Home › Forums › JAMES ROGUSKI › James Roguski Discover more from James Roguski Exposing MDM (Mis-Information, Dis-Information and Mal-Information) Over 70,000 subscribers Type your email… Continue reading Sign in Evidence from the Pfizer Papers Far from being “safe and effective,” in the clinical trials, more people who received the Pfizer “vaccine” DIED than those who were in the placebo group. The “vaccine” was NEVER shown to save lives.
- This topic is empty.
-
AuthorPosts
-
2025-01-01 at 18:37 #458897
 Nat QuinnKeymaster
Nat QuinnKeymasterEvidence from the Pfizer Papers
Far from being “safe and effective,” in the clinical trials, more people who received the Pfizer “vaccine” DIED than those who were in the placebo group. The “vaccine” was NEVER shown to save lives.

FOR COMPLETE DETAILS: NotSafeAndNotEffective

Why did Pfizer want to hide the details of their clinical trial for 75 years?
Maybe it was because more people who received Pfizer’s BNT162b2 nRNA “vaccine” DIED when compared to those who received the placebo.
In the Pfizer/BioNTech Clinical Trial (C4591001) the “vaccine” DID NOT SAVE LIVES.
WE WERE ALL LIED TO

Dr. Naomi Wolf and many others have analyzed these documents and published a series of reports (See below)

https://x.com/wideawake_media/status/1847221093595062305
Dr. Robert Chandler – National Citizens Inquiry Testimony

https://rumble.com/v503k19-dr.-robert-chandler-may-31-2024-regina-saskatchewan.html?start=1020
Naomi Wolf

https://rumble.com/v5iclf9-dr.-naomi-wolf-reveals-shocking-details-in-the-pfizer-papers.html
Naomi Wolf

https://freedomlibrary.hillsdale.edu/programs/cca-iv-big-pharma/what-s-in-the-pfizer-documents

LIE #1:
July 27, 2020 to November 14, 2020
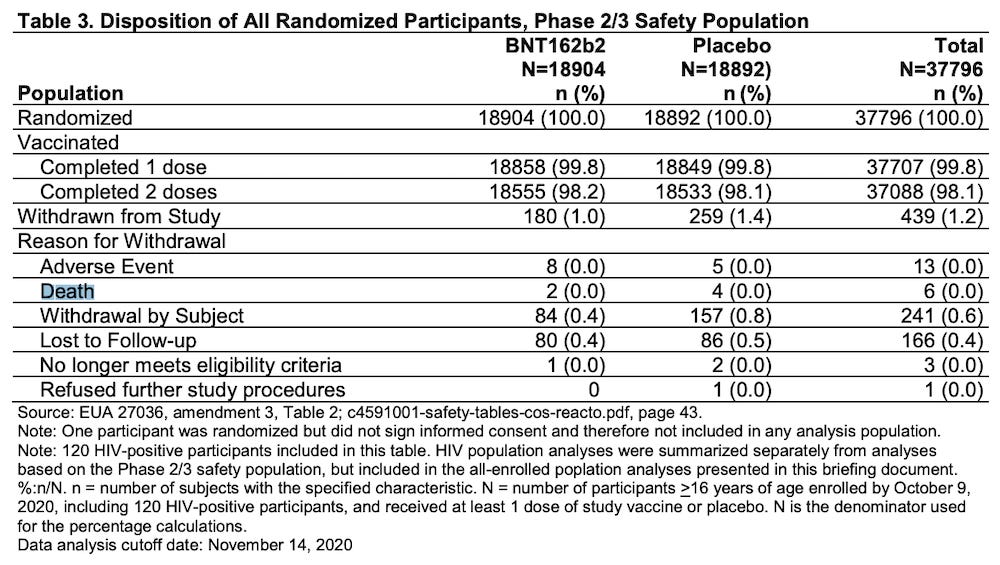
Pfizer concealed at least 2 deaths in the “vaccine” arm of their clinical trial that should have been revealed BEFORE the FDA granted an Emergency Use Authorization on December 11, 2020.

BLINDED PLACEBO-CONTROLLED PERIOD TO EUA APPLICATION DATA COLLECTION CUT-OFF
Pfizer submitted the Emergency Use Application on November 20, 2020 with the following data (THEY LIED):
https://www.fda.gov/media/144416/download (page 19)
The cutoff period for data collection was November 14, 2020, at which time the DEATH TOLL was far different than what Pfizer claimed in their Application for Emergency Use Authorization.
Pfizer hid vaccine deaths, research team alleges
During the clinical trials for its COVID-19 vaccine, pharmaceutical giant Pfizer appears to have hidden two deaths.
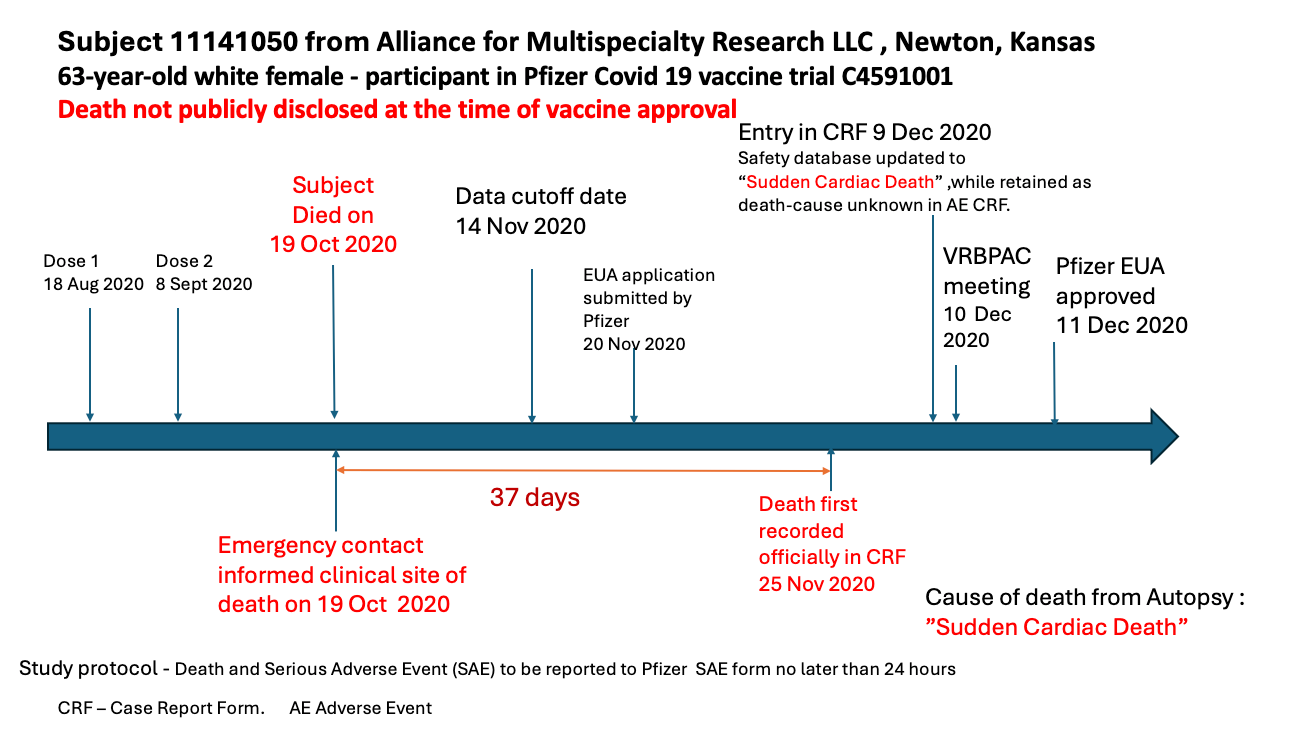
According to Dr. Jeyanthi Kunadhasan, an anesthetist and perioperative physician in Australia; who was part of the team, the study protocol required that any “death or serious adverse effect” had to be reported within 24 hours. In the Kansas case that did not happen for 37 days.
The Kansas case was a 63-year-old woman who had her first dose of the Pfizer mRNA vaccine on August 18, 2020, and a second dose on September 8, 2020. She died on October 19, 2020, and her emergency contact immediately informed the clinical site — Alliance for Multispecialty Research LLC, in Newton, Kansas. Thirty-seven days later, on November 25, 2020 — 11 days after the data reporting cutoff date — the death was finally recorded in a “case report form.”
Five days after the emergency use application was submitted to the Food and Drug Administration by Pfizer.
The participant’s death was not reported in the trial results in the prestigious New England Journal of Medicine or to the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), which approved the EUA.
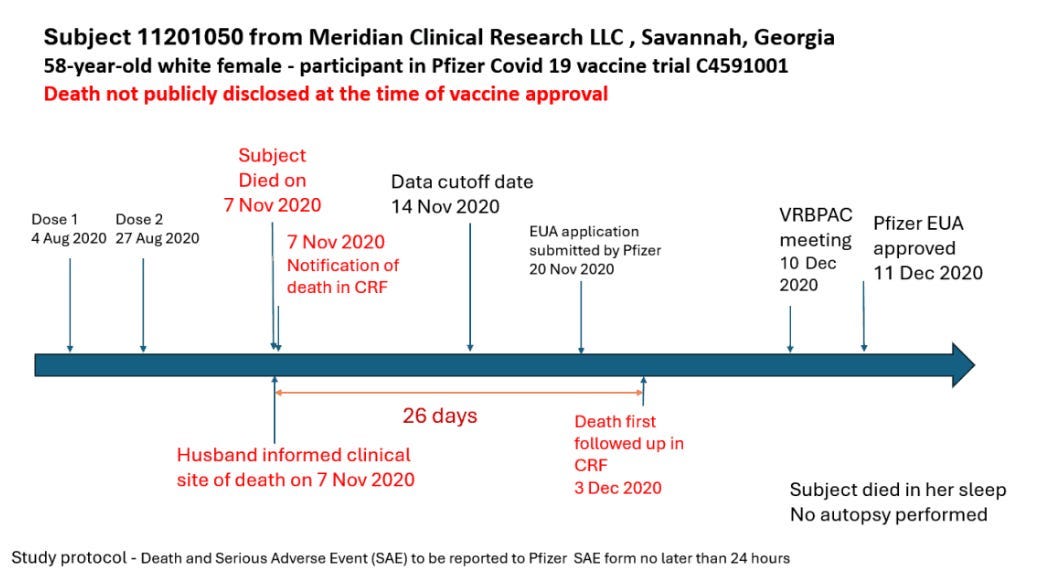
The second hidden death was a 58-year-old woman in Georgia.
She received her first dose on August 4, 2020, and a second on August 27, 2020. The woman died in her sleep on November 7, 2020, and her husband immediately informed the clinical site. The death was not added to the data for 26 days and first followed up on December 3, 2020 — again well after the Nov. 14 data cutoff date.
The reports shown to [the Vaccines and Related Biological Products Advisory Committee] VRBPAC and in the New England Journal of Medicine stated there were only six deaths in the trial, four in the placebo group and two in the vaccinated group, supposedly showing that the vaccine worked and reduced the risk of death.
However there were actually four in each group.
The bottom line?
Kunadhasan believes Pfizer knew the vaccine didn’t work and could cause heart problems — even before it was approved.
https://BehindTheFDAcurtain.substack.com/p/pfizer-hid-vaccine-deaths-research
Letter to Texas Attorney General Ken Paxton: Vaccinated Deaths in Pfizer’s COVID Vaccine Clinical Trial Not Disclosed to FDA with EUA Data.
I wish to highlight two undisclosed deaths of American trial participants in the BNT162b2-vaccinated arm of Pfizer’s clinical trial. Pfizer’s nondisclosure of these deaths occurred before Pfizer’s data cut-off date for its EUA submission to the FDA. (Michels et al., 2023)
Polack et al. released their findings, “Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine,” on December 10, 2020, one day before the FDA issued Pfizer’s [Emergency Use Authorization] EUA.
Polack, Fernando, et al. “Safety and Efficacy of the BNT162B2 Mrna Covid-19 Vaccine.” New England Journal of Medicine, nejm.org, 10 Dec. 2020, http://www.nejm.org/doi/full/10.1056/NEJMoa2034577
The Polack paper disclosed six deaths — two in the BNT162b2 arm and four in the placebo arm. Both the journal article and the EUA approval documentation showed the six deaths during the period of July 27, 2020, till November 14, 2020.
This letter will demonstrate that Pfizer-BioNTech had records showing eight deaths, four in the BNT162b2 arm and four in the placebo arm, that Pfizer should have been disclosed to the FDA.
The September 2023 Pfizer-BioNTech data released by the FDA introduced a document named “125742_S1_M5_5351_c4591001-interim-mth6-narrative-sensitive.pdf,”
Pfizer. “125742_S1_M5_5351_c4591001-Interim-Mth6-Narrative-Sensitive.Pdf.” Public Health and Medical Professionals for Transparency, phmpt.org, 1 Sept. 2023, phmpt.org/wp-content/uploads/2023/09/125742_S1_M5_5351_c4591001-interim-mth6-narrative-sensitive.pdf.
which included information revealing that Pfizer-BioNTech was, in fact, informed of two additional deaths in the BNT162b2 arm of the trial well before the EUA data cut-off date, and that Pfizer-BioNTech did not disclose those deaths to the FDA.
If the deaths had been disclosed in the EUA submission, they would have shown that the BNT162b2 mRNA COVID vaccine intervention did not reduce deaths.
Subject 11141050 from Alliance for Multispecialty Research LLC , Newton, Kansas, in the vaccinated arm of the study, died on October 19, 2020. Contrary to Pfizer-BioNTech’s clinical trial protocol, neither Polack et al., nor the EUA submission documentation, nor the VRBPAC meeting on December 10, 2020, disclosed this patient’s death.
The death occurred well before the data cut-off date of November 14, 2020.
The public lacks access to any of the original clinical trial records, specifically Pfizer Safety’s Vaccine SAE Reporting Form for subjects. However, from the patient narratives (Pfizer, 2023, p. 71), it is evident that the emergency contact confirmed on the day of death (October 19, 2020) that the subject had died.
As this death occurred well before the data cut-off date of November 14, 2020, and was known to Pfizer on November 25, 2020, there was ample opportunity to disclose this subject’s death, and possibly the autopsy results, at the December 10, 2020, VRBPAC meeting.
Subject 11201050, from Meridian Clinical Research LLC, Savannah, Georgia, died on November 7, 2020. The patient narratives explicitly state that the clinical site received notification of the subject’s death on November 7, 2020, from her husband.(Pfizer 2023, p. 75). This information is further supported by documentation found in that patient’s CRF clearly stating that the death notification occurred on November 7, 2020.[21]
Given these established facts, it is puzzling that the death of this subject was not included with the other data to the FDA when seeking EUA. Moreover, it was not disclosed by the clinical trial investigators to the regulators during the December 10, 2020, VRBPAC meeting (Vaccines and Related Biological Products Advisory Committee, 2020). This is particularly perplexing as the death occurred and was acknowledged as known before the November 14, 2020, data cut-off date.
We have documentation in the publicly available Pfizer clinical trial documents that confirms the patients’ loved ones promptly communicated the subjects’ deaths to the clinical trial sites. However, in violation of legal requirements, the regulatory authorities were apparently not informed of these deaths within the specified time frame. The critical time period under scrutiny is the issuance of the EUA on December 11, 2020, which relied upon the clinical trial data collected through November 14, 2020.
During the December 10, 2020, VRBPAC meeting, one reason cited for vaccine approval was “the known and potential benefits of the vaccine outweigh the known and potential risks of the vaccine when used for active immunization to prevent COVID-19 caused by SARS-CoV-2 in individuals 16 years of age and older” (Vaccines and Related Biological Products Advisory Committee, 2020).
Notably, the omission of the two deaths from the vaccinated arm of the study at this critical juncture of EUA issuance raises substantial concerns about the overall safety reporting of Pfizer’s clinical trial.

LIE #2:
July 27, 2020 to March 13, 2021
In the Pfizer clinical trials, more people who received the Pfizer “vaccine” DIED than those who were in the placebo group.
The “vaccine” was NEVER shown to save lives.

 Forensic Analysis Of The 38 Subject Deaths In The 6 Month Interim Report Of The Pfizer Biontech Bnt162b2 Mrna Vaccine Clinical Trial887KB ∙ PDF file
Forensic Analysis Of The 38 Subject Deaths In The 6 Month Interim Report Of The Pfizer Biontech Bnt162b2 Mrna Vaccine Clinical Trial887KB ∙ PDF file
Pfizer/BioNTech Clinical Trial C4591001
The brilliant Dr Jeyanthi Kunadhasan testifies before an Australian Senate Committee that in Pfizer’s own clinical trial there were more deaths in the vaccinated group than the placebo group.

https://x.com/craigkellyXXX/status/1801258124436971713
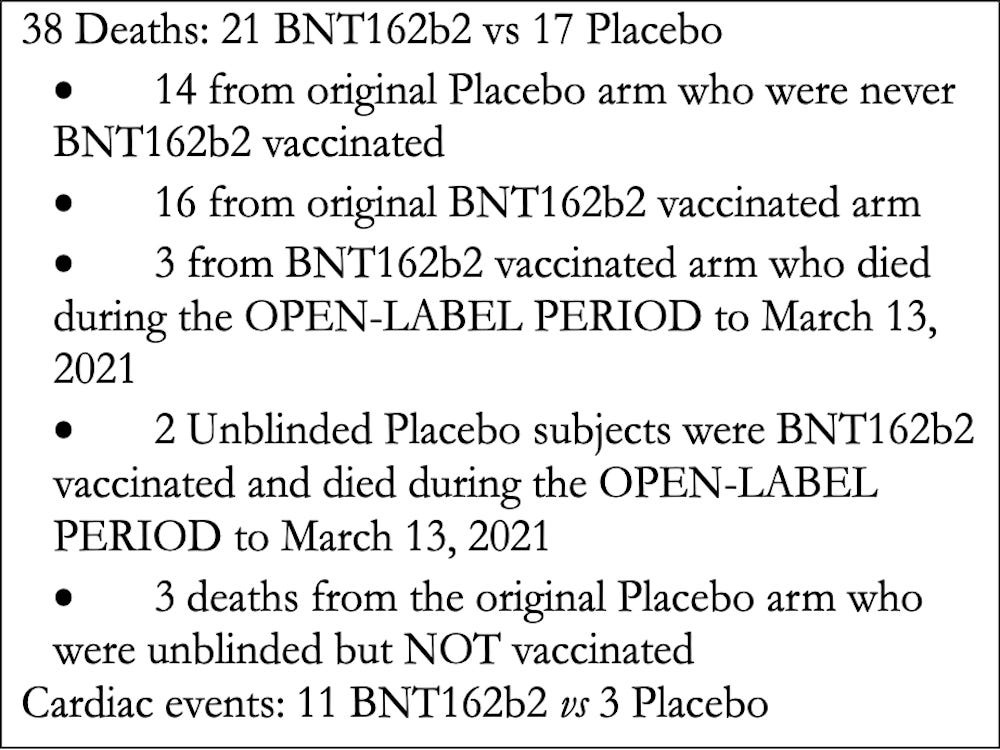
The Pfizer Papers revealed the following:
SUMMARY OF DEATHS IN 6-MONTH REPORTING PERIOD: July 27, 2020 to March 13, 2021
Deaths reported among those who received the Pfizer BNT162b2 “vaccine”:
There were a total of 19 deaths among those who received 2 injections of BNT162b2.
There were also 2 additional deaths among those who were originally in the placebo group but then received 2 injections plus a booster after the clinical trial was unblinded.
PLEASE NOTE:
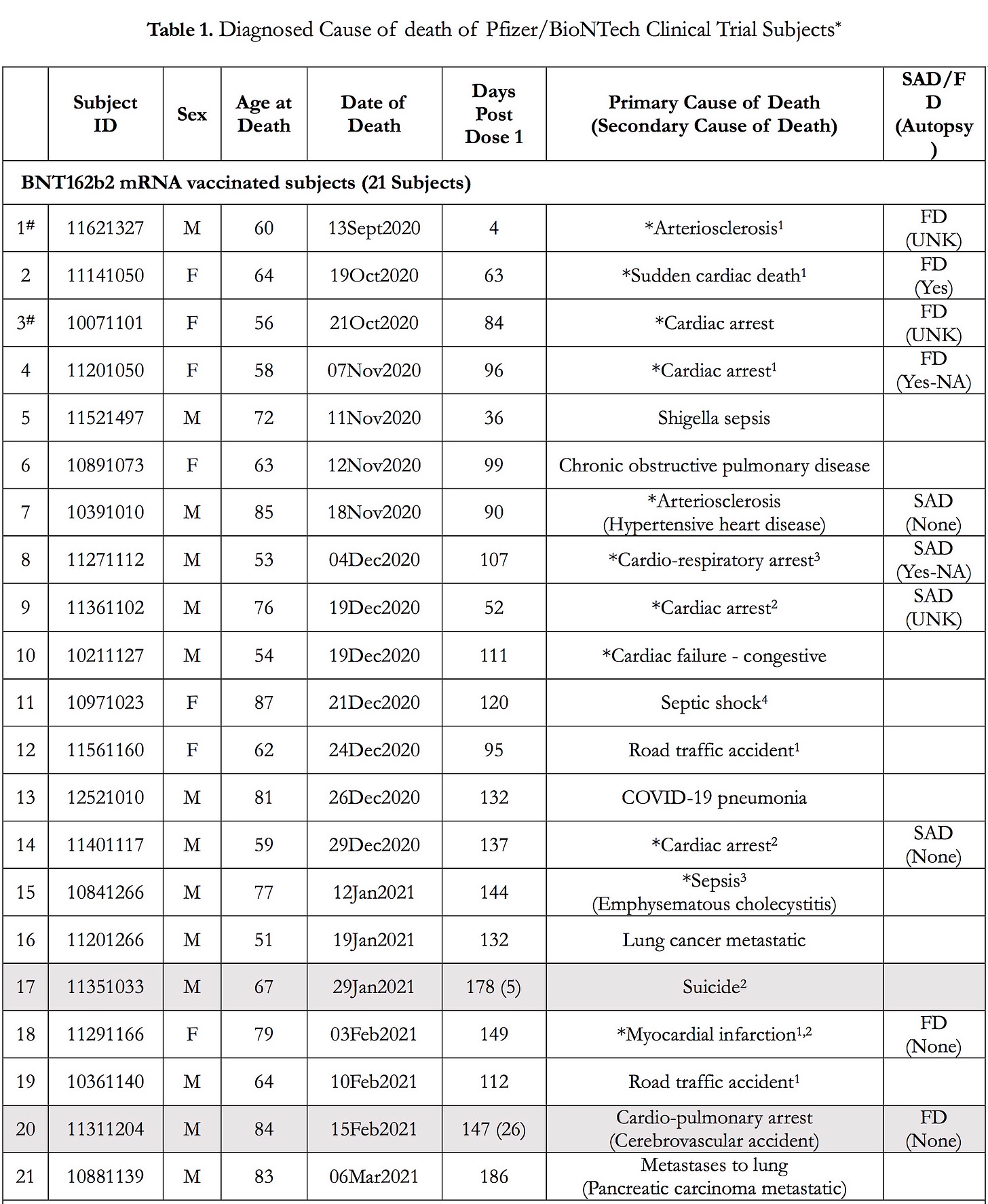
There were actually 8 deaths that occurred among those who received the Pfizer “vaccine” before the FDA granted an Emergency Use Authorization on December 11, 2020, but only two of the deaths were reported by Pfizer in their EUA application (see Pfizer document above and 1# and 3# below).
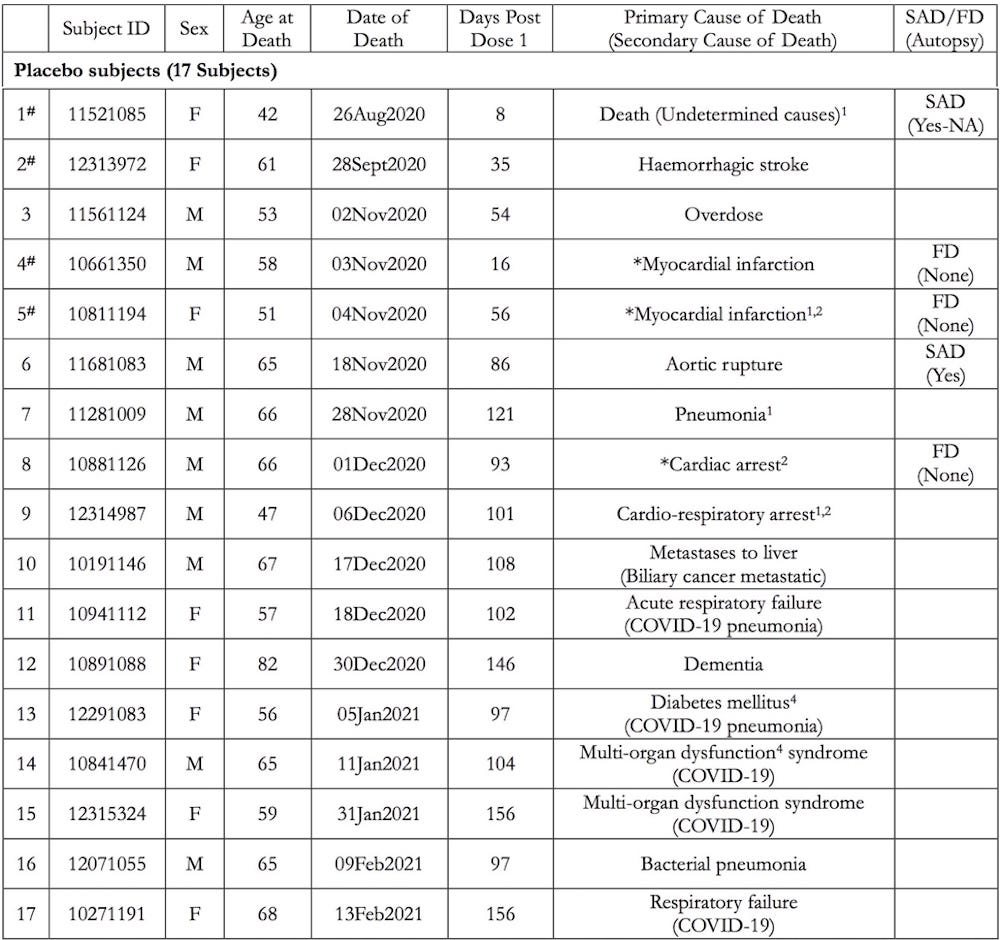
There were 17 Deaths in the placebo arm of the clinical trial:

CONCLUSIONS
- The C4591001 placebo-controlled randomized clinical trial of 22,030 vaccinated and 22,030 placebo subjects was the world’s only opportunity for an unbiased evaluation of the Pfizer/BioNTech BNT162b2 vaccine.
- Unblinding of placebo subjects starting in Week 20 terminated the placebo-controlled clinical trial, thereby ending all unbiased evaluation of possible adverse event signals.
- The mRNA-LNP platform is novel, not previously phase 2/3 tested in humans, and the toxicity of PP-Spike protein was unknown. Taken together, a 20-weeks placebo-controlled clinical trial is NOT sufficient to identify any except for the most common safety concerns.
- The number of all-cause deaths is NOT decreased by BNT162b2 vaccination.
- Of the 38 deaths reported in the 6-Month Interim Report of Adverse Events, 21 BNT162b2 vaccinated subjects died compared to 17 placebo subjects.
- Delayed reporting of the subject deaths into the Case Report Form, which was in violation of the trial protocol, allowed the EUA to proceed unchallenged.
- The number of subject deaths was 17% of the expected number, based on age-adjusted US mortality. One possible explanation could lie in the 395 subjects that were “Lost to Follow- up”.
- There was a 3.7-fold increase in cardiac events in subjects who received the BNT162b2 vaccine versus the placebo.
- Of the 15 subjects who were Sudden Adult Deaths (SAD) or Found Dead (FD), 12 died of a cardiac event, 9 of whom were vaccinated.
- The cardiac adverse event signal was obscured by delays in reporting the accurate date of subject death that was known to Pfizer/BioNTech in the subject’s Narrative Report.
https://ijvtpr.com/index.php/IJVTPR/article/view/86/224
https://pdfs.semanticscholar.org/1d79/a14307e35646b8fd9209fc8a17e12d2d6ca9.pdf
Additional Observations:
- Early cancer signal?
We observe that there were 2 deaths from metastatic cancer in the vaccine group while only 1 in the placebo group. Could this indicate an early signal that identifies accelerated outcomes for exisiting cancers or the new phenomenon of “turbo-cancers”?
In our research, we find evidence of this possible signal in our research on malignant neoplasms deaths in the UK. The analysis of deaths for all malignant neoplasms can be found here, and the analysis of individual malignant neoplasm causes (including cancers without site specification), here. - Road traffic accidents?
We observe that there were 2 deaths from road traffic accidents in the vaccine group while none in the placebo group. Is this a signal or spurious event? - Sepsis-related deaths?
We observe that there were 3 sepsis-related deaths in the vaccine group while none in the placebo group. Is this a signal or spurious event? - All-cause mortality signal?
We observe that there were 21 [19?] deaths in the vaccine group while 17 in the placebo group, corresponding to excess deaths around 23% [11.7%?]. Is this an early signal that corroborates the excess mortality we observe in the nations we’ve investigated? (link to our excess mortality analysis)

References:
2.7.4 STN Summary Clinical Safety
https://phmpt.org/wp-content/uploads/2021/12/STN-125742_0_0-Section-2.7.4-summary-clin-safety.pdf
6-Month Interim Report of Adverse Events C4591001
16.1.7.1 Listing of Randomization Scheme and Actual Vaccine Received – All Subjects ≥16 Years of Age
16.2.1.1 Listing of Subjects Discontinued From Vaccination and/or From the Study – All Subjects ≥16 Years of Age
16 2 1 1 Listing Of Subjects Discontinued From Vaccination And Or From The Study4.19MB ∙ PDF file
A Phase 1/2/3 Study to Evaluate the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates Against COVID-19 in Healthy Individuals
A Phase 1 2 3 Placebo Controlled Randomized Observer Blind Dose Finding Study1.31MB ∙ PDF file
COURT DOCUMENTS:
Find out what was in the documents that Pfizer and Moderna tried to keep hidden from the public.
The US Food and Drug Administration (FDA) attempted to delay the public release of Pfizer’s COVID-19 vaccine safety data for 75 years, despite approving the injection after only 108 days of safety review on December 11th, 2020.
On January 6, 2022 United States District Judge Mark Pittman of the United States District Court in the Northern District of Texas ordered the release of the Pfizer clinical trial documents (55,000 pages per month). They released 12,000 pages by the end of January, 2022.
Since then, PHMPT has posted all of the documents on its website.
December 6, 2024:
FDA must disclose more COVID-19 vaccine records, US judge rules
A federal judge has ordered the U.S. Food and Drug Administration to publicly disclose more information underpinning its authorization of COVID-19 vaccines, after failing to persuade the court to end the public records lawsuit.
In a ruling, on Friday, U.S. District Judge Mark Pittman in Fort Worth, Texas, ordered the agency to produce its “emergency use authorization” file to a group of scientists who wanted to see licensing information that the FDA relied on to approve the Pfizer-BioNTech coronavirus vaccine.
“The COVID-19 pandemic is long passed and so has any legitimate reason for concealing from the American people the information relied upon by the government in approving the Pfizer vaccine,” wrote Pittman.
The lawsuit, filed in late 2021, attracted attention after the FDA said it could take decades to process and disclose records to Public Health and Medical Professionals for Transparency, the group that brought the case.
The FDA declined to comment.
The Covid-19 pandemic is long passed and so has any legitimate reason for concealing from the American people the information relied upon by the government in approving the Pfizer Vaccine.
It is ORDERED that the FDA shall produce the responsive EUA file on or before June 30, 2025.
Mark T. Pittman
United States District Judge


PHMPT.org (Clinical Trial Data)
This nonprofit, made up of public health professionals, medical professionals, scientists, and journalists exists solely to obtain and disseminate the data relied upon by the FDA to license COVID-19 vaccines. The organization takes no position on the data other than that it should be made publicly available to allow independent experts to conduct their own review and analyses.
https://phmpt.org/pfizer-16-plus-documents/
https://phmpt.org/pfizer-12-15-documents/
https://phmpt.org/moderna-documents/
https://www.globalresearch.ca/wp-content/uploads/2023/05/pfizer-report.pdf

The Daily Clout’s 100+ Reports
3,200 medical professionals, lawyers and scientists make up the investigatory group that wrote the papers after reading every FDA dump throughout 2022 and 2023 until the final dump was made.
One of the most interesting reports:
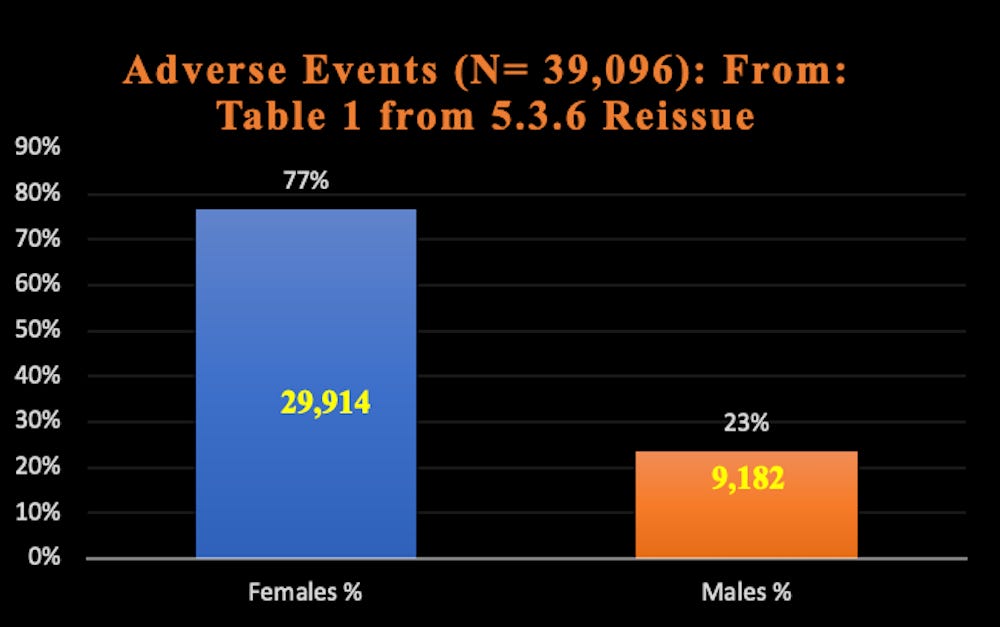
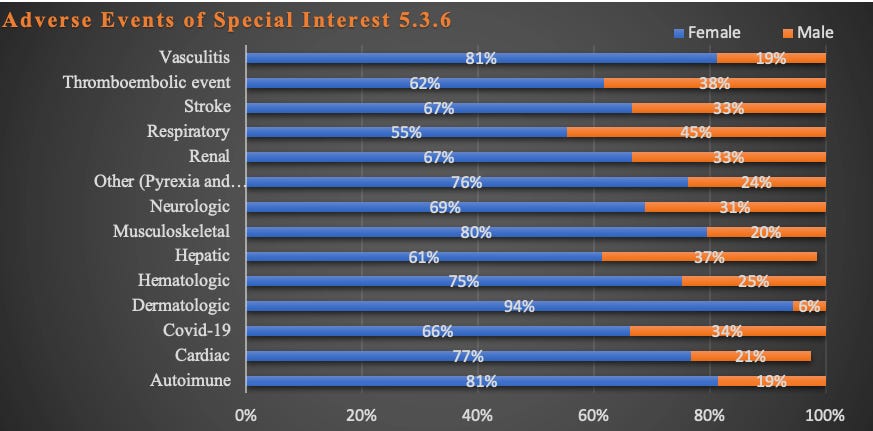
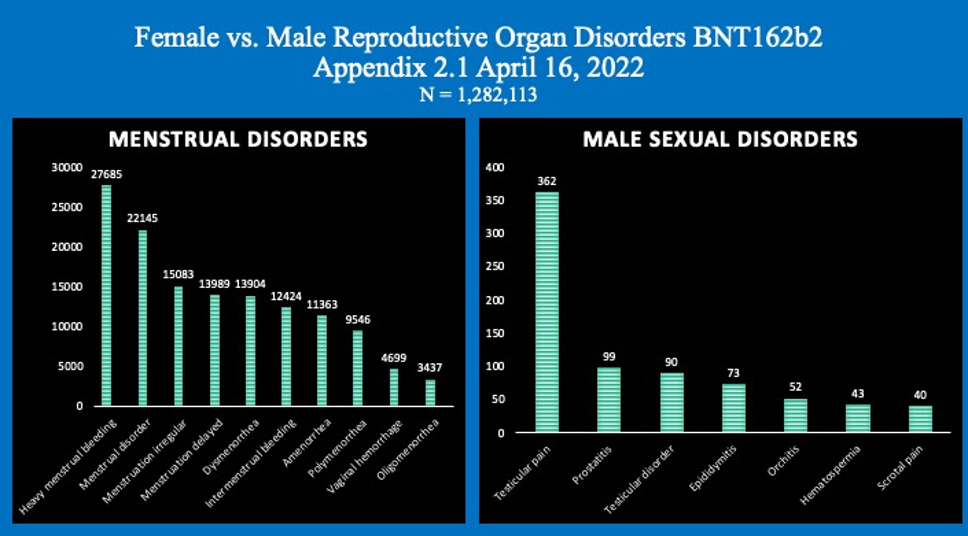
Report 38: Women Have Two and a Half Times Higher Risk of Adverse Events Than Men. Risk to Female Reproductive Functions Is Higher Still [August 20, 2022]
https://dailyclout.io/pfizer-and-moderna-reports/
- Report 1: Where Are Pfizer’s 20,000 Missing Patients? [March 31, 2022]
- Report 2: 136 Deaths and 1625 Serious Cases of ‘Ineffectiveness’ Revealed [April 8, 2022]
- Report 3: Phase 1 /2 Study of COVID-19 RNA Vaccine BNT162b1 in Adults: Key Processes Missing [April 30, 2022]
- Report 4: Not available
- Report 5: Pfizer mRNA Construct – Why Spike Protein Causes Disease [May 13, 2022]
- Report 6: Not available
- Report 7: COVID-19 Vaccines and Pregnancy: Risky Business [May 18, 2022]
- Report 8: Not available
- Report 9: Not available
- Report 10: Even Big Pharma CEOs Recognized That Not Everyone Could be Vaccinated – So Why the Mandates? {May 3, 2022]
- Report 11: Pfizer Vaccine – FDA Fails to Mention Risk of Heart Damage in Teens [April 7, 2022]
- Report 12: Secret Documents: How Pfizer Covered Up a Flood of Adverse Events [April 5, 2022]
- Report 13: MISSING – 50 Pregnant Women From Pfizer Clinical Trials [May 3, 2022]
- Report 14: Were We Lied to by the FDA? [May 2, 2022]
- Report 15: Adverse Events Rise in Babies Breastfed by Vaccinated Mothers [May 23, 2022]
- Report 16: MicroRNA, the Hidden RNA in the Pfizer Vaccine [May 20, 2022]
- Report 17: Why COVID-19 Vaccine Consent Must Be Informed [May 18, 2022]
- Report 18: Vaccine ‘Shedding’: Can This Be Real After All? [May 13, 2022]
- Report 19: Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine [May 12, 2022]
- Report 20: Not available
- Report 21: What Did Pfizer Know, and When Did They Know It? Neurological Harms Concealed [May 2, 2022]
- Report 22: Effects of N1-Methyl-Pseudouridine in the Pfizer mRNA Vaccine [May 11, 2022]
- Report 23: Harmful Cytokines From Vaccinated Mothers Passed to Breastfed Infants – Report [June 3, 2022]
- Report 24: Dr. Fernando Polack – Real Person or Ghost? [June 6, 2022]
- Report 25: Strokes – What Did Pfizer Know, and When Did They Know It? [June 13, 2022]
- Report 26: Proof the TrialMax App Unequivocally Contributed to Pfizer’s Deception of Safety [June 23, 2022]
- Report 27: Even Big Pharma CEOs Recognized Not Everyone Could be Vaccinated. So Why the Mandates? [June 23, 2022]
- Report 28: Vaccine Trials for Infants and Children Show Little to No Benefit [June 27, 2022]
- Report 29: Did Pfizer and the FDA Conceal An Existing Remedy for COVID? [June 29, 2022]
- Report 30: Inconsistencies in Pfizer Clinical Trials Are Surfacing [June 29, 2022]
- Report 31: Pfizer-BioNTech “Equivalent” Half Truths or a “Lot” of Lies? [June 29, 2022]
- Report 32: If Pfizer Controlled the ‘Data’ They Controlled the Outcome [June 28, 2022]
- Report 33: Pfizer’s New Two-in-One COVID-19 Booster: Are We the Clinical Trial? [July 19, 2022]
- Report 34: Understanding C-19 Vaccine Efficacy Clinical Trial in Lay Terms [July 21, 2022]
- Report 35: Pfizer Evidence So Far: Coverups, Heart Damage, and More [July 30, 2022]
- Report 36: Pfizer Used Dangerous Assumptions, Rather than Research, to Guess at Outcomes [August 1, 2022]
- Report 37: Pfizer, FDA, CDC Hid Proven Harms to Male Sperm Quality, Testes Function, from mRNA Vaccine Ingredients [August 16, 2022]
- Report 38: Women Have Two and a Half Times Higher Risk of Adverse Events Than Men. Risk to Female Reproductive Functions Is Higher Still [August 20, 2022]
- Report 39: Despite Incomplete Safety Trials, the Food and Drug Administration (FDA) Grants Full Approval to Pfizer-BioNTech’s COMIRNATY® for Adolescents 12-15 Years of Age [September 4, 2022]
- Report 40: Data Do Not Support Safety of mRNA COVID Vaccination for Pregnant Women [September 16, 2022]
- Report 41: 2021 CDC and FDA Misinformation – Retroactive Editing, Erroneous Spontaneous Abortion Rate Calculation, Obfuscation in the New England Journal of Medicine [September 26, 2022]
- Report 42: Pfizer’s EUA Granted Based on Fewer Than 0.4% of Clinical Trial Participants. FDA Ignored Disqualifying Protocol Deviations to Grant EUA. [September 26, 2022]
- Report 43: Twenty-Two Cases of Rare Myocarditis by February 2021, Yet Pfizer Said No “New Safety Issues.” FDA Waits Until June 25, 2021, to Include Myocarditis Risk in Fact Sheets. [August 26, 2022]
- Report 44: Is mRNA-LNP Vaccine-Induced Immunity Inheritable? A Preprint Study Shows It Is. [October 6, 2022]
- Report 45: Failure of Serialization By Pfizer Flouted Established Pharma Industry Rules [October 13, 2022]
- Report 46: How Many Pregnant Women Received LNP/mRNA via COVID-19 Vaccine During the Year 2021? Only Estimates Are Available. [November 26, 2022]
- Report 47: Blood System-Related Adverse Events Following Pfizer COVID-19 mRNA Vaccination [November 30, 2022]
- Report 48: VAERS – 76% of Vaccine-Related Miscarriages from the Past 30 Years Occurred Once Pregnant Women Started Receiving COVID-19 Vaccines [December 1, 2022]
- Report 49: Clotting System-Related Adverse Events Following Pfizer COVID-19 mRNA Vaccination [December 14, 2022]
- Report 50: 20% of Post-Jab Strokes Fatal in the 90 Days Following Pfizer COVID mRNA Vaccine Rollout [December 26, 2022]
- Report 51: Liver Adverse Events – Five Deaths Within 20 Days of Pfizer’s mRNA COVID Injection. 50% of Adverse Events Occurred Within Three Days. [January 11, 2023]
- Report 52: Nine Months Post-COVID mRNA “Vaccine” Rollout, Substantial Birth Rate Drops in 13 European Countries, England/Wales, Australia, and Taiwan. [January 16, 2023]
- Report 53: 77% of Cardiovascular Adverse Events from Pfizer’s mRNA COVID Shot Occurred in Women, as Well as in People Under Age 65. Two Minors Suffered Cardiac Events. [January 19, 2023]
- Report 54: Infants and Children Under 12 Given the Pfizer mRNA COVID “Vaccine” Seven Months BEFORE Pediatric Approval. 71% of Adverse Event Cases Classified as Serious. [January 31, 2022]
- Report 55: Is Pfizer Trustworthy? – The Company Has a Long History of Legal Fines, Penalties, and Serious Violations. [February 10, 2022]
- Report 56: Histopathology Series Part 1 – Autopsies Reveal Medical Atrocities of Genetic Therapies Being Used Against a Respiratory Virus [February 15, 2023]
- Report 57: 542 Neurological Adverse Events, 95% Serious, in First 90 Days of Pfizer mRNA Vaccine Rollout. 16 Deaths. Females Suffered AEs More Than Twice As Often As Males. [February 20, 2023]
- Report 58: Histopathology Series Part 2 – “Autopsies Reveal Medical Atrocities of Genetic Therapies Being Used Against a Respiratory Virus” [March 3, 2023]
- Report 59: The Flawed Trial of Pfizer’s COVID-19 mRNA “Vaccine.” 90% of Original Placebo Group Received at Least One mRNA Injection by March 2021 [March 6, 2023]
- Report 60: 449 Patients Suffer Bell’s Palsy Following Pfizer mRNA COVID Vaccination in Initial Three Months of Rollout. A One-Year-Old Endured Bell’s Palsy After Unauthorized Injection. [March 8, 2023]
- Report 61: Histopathology Series Part 3 – Ute Krüger, MD, Breast Cancer Specialist, Reveals Increase in Cancers and Occurrences of “Turbo Cancers” Following Genetic Therapy “Vaccines” [March 10, 2023]
- Report 62: Acute Kidney Injury and Acute Renal Failure Following Pfizer mRNA COVID Vaccination. 33% of Patients Died. Pfizer Concludes, “No New Safety Issue.” [March 17, 2023]
- Report 63: In Q3 2022, Pfizer Wrote Off $450 Million of Expired or Expiring COVID-19-Related Inventory. [February 27, 2023]
- Report 64: Histopathology Series Part 4a – Re-Humanizing Data Using “Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series.” [March 27, 2023]
- Report 65: In the First Three Months of Pfizer’s mRNA “Vaccine” Rollout, Nine Patients Died of Anaphylaxis. 79% of Anaphylaxis Adverse Events Were Rated as “Serious.” [March 31, 2023]
- Report 66: 1,077 Immune-Mediated/Autoimmune Adverse Events in First 90 Days of Pfizer mRNA “Vaccine” Rollout, Including 12 Fatalities. Pfizer Undercounted This Category of Adverse Events by 270 Occurrences. [April 13, 2023]
- Report 67: Histopathology Series Part 4b – Rhabdomyolysis – a.k.a., “Jellied Muscle” – After mRNA Gene Therapy Injections [April 14, 2023]
- Report 68: 34 Blood Vessel Inflammation, Vasculitis, Adverse Events Occurred in First 90 Days After Pfizer mRNA “Vaccine” Rollout, Including One Fatality. Half Had Onset Within Three Days of Injection. 81% of Sufferers Were Women. [April 25, 2023]
- Report 69: BOMBSHELL – Pfizer and FDA Knew in Early 2021 That Pfizer mRNA COVID “Vaccine” Caused Dire Fetal and Infant Risks, Including Death. They Began an Aggressive Campaign to Vaccinate Pregnant Women Anyway. [April 29, 2023]
- Report 70: Histopathology Series Part 4c – Autoimmunity: A Principal Pathological Mechanism of COVD-19 Gene Therapy Harm (CoVax Diseases) and a Central Flaw in the LNP/mRNA Platform [May 5, 2023]
- Report 71: Musculoskeletal Adverse Events of Special Interest Afflicted 8.5% of Patients in Pfizer’s Post-Marketing Data Set, Including Four Children and One Infant. Women Affected at a Ratio of Almost 4:1 Over Men. [May 15, 2023]
- Report 72: “Other AESIs” Included MERS, Multiple Organ Dysfunction Syndrome (MODS), Herpes Infections, and 96 DEATHS. 15 Patients Were Under Age 12, Including Six Infants. [May 30, 2023]
- Report 73: Pfizer Knew by November 2020 That Its mRNA COVID Vaccine Was Neither Safe Nor Effective. Here Is What Pfizer’s Employees and Contractors Knew and When They Knew It. [May 31, 2023]
- Report 74: Lipid Nanoparticles Corrupt Nature [June 5, 2023]
- Report 75: mRNA COVID “Vaccines” Have Created a New Class of Multi-Organ/System Disease: “CoVax Disease.” Children from Conception on Suffer Its Devastating Effects. – Histopathology Series – Part 4d [July 3, 2023]
- Report 76: Pfizer Had Data to Announce Its COVID-19 Vaccine’s Alleged “Efficacy” in October 2020. Why Did Pfizer Delay the Announcement Until November 9, 2020, Six Days After the 2020 U.S. Presidential Election? [July 10, 2023]
- Report 77: Women Suffered 94% of Dermatological Adverse Events Reported in First 90 Days of Pfizer COVID “Vaccine” Rollout. 80% of These Adverse Events Were Categorized As “Serious.” [July 11, 2023]
- Report 78: Thirty-Two Percent of Pfizer’s Post-Marketing Respiratory Adverse Event Patients Died, Yet Pfizer Found No New Safety Signals. [August 4, 2023]
- Report 79: mRNA COVID Vaccine-Induced Myocarditis at One Year Post-Injection: Spike Protein, Inflammation Still Present in Heart Tissue. [August 7, 2023]
- Report 80: Moderna mRNA COVID-19 Injection Damaged Mammals’ Reproduction: 22% Fewer Pregnancies; Skeletal Malformations, Pain, Nursing Problems in Pups. FDA Knew, Yet Granted EUA. [August 11, 2023]
- Report 81: Summary of 2.4 Nonclinical Overview – Pfizer mRNA COVID-19 Vaccine, BNT162b2 [August 29, 2023]
- Report 82: Pfizer Clinical Trial Subject Dies Soon After Receiving One Dose of Moderna COVID Vaccine. Forty-Two Days Post-Mortem, Pfizer Staff Seem Unaware He Is Dead. [August 23, 2023]
- Report 83: 23% of Vaccinated Mothers’ Fetuses or Neonates Died. Suppressed Lactation and Breast Milk Discoloration Reported. [September 1, 2023]
- Report 84: War Room/DailyClout Research Team Breaks Huge Story: More Cardiovascular Deaths in Vaxxed Than Unvaxxed; Pfizer Did Not Report Adverse Event Signal; Death Reporting Delays Favored Pfizer/Vaccinated. [September 5, 2023]
- Report 85: “The Underlying Pathology of Spike Protein Biodistribution in People That Died Post COVID-19 Vaccination” – Dr. Arne Burkhardt [September 10, 2023]
- Report 86: Pfizer’s Clinical Trial ‘Process 2’ COVID Vaccine Recipients Suffered 2.4X the Adverse Events of Placebo Recipients; ‘Process 2’ Vials Were Contaminated with DNA Plasmids. [October 6, 2023]
- Report 87: In Early 2021, Pfizer Documented Significant Harms and Deaths Following Vaccination with Its mRNA COVID Vaccine. The FDA Did Not Inform the Public. [October 10, 2023]
- Report 88: 2.5 Months After COVID Vaccine Rollout, Pfizer Changed Criteria for ‘Vaccination Failure,’ Causing 99% of Reported Cases to Not Meet That Definition. 3.9% of Reported ‘Lack of Efficacy’ Cases Ended in Death in First 90 Days of Public Vaccine Availability. [October 11, 2023]
- Report 89: BOMBSHELL – War Room/DailyClout Researchers Find Pfizer Delayed Recording Vaccinated Deaths at Critical Juncture of EUA Process. Improper Delays in Reporting Deaths in the Vaccinated Led FDA to Misstate Vaccine’s Effectiveness, Influenced EUA Grant Decision. [October 16, 2023]
- Report 90: Pfizer’s ‘Post-Marketing Surveillance’ Shows mRNA-Vaccinated Suffered 1000s of COVID Cases in 1st 90 Days of Vaccine Rollout. Most Infections in the Vaccinated Categorized as ‘Serious Adverse Events.’ [November 9, 2023]
- Report 91: FDA Based Moderna’s mRNA COVID Vaccine Approval on Test of a Completely Different Non-COVID Vaccine. Only Males Included in Test. [November 14, 2023]
- Report 92: 100s of Possible Vaccine-Associated Enhanced Disease (VAED) Cases in First 3 Months of Pfizer’s mRNA COVID Vaccine Rollout, Yet Public Health Spokespeople Minimized Their Severity by Calling Them “Breakthrough Cases.” [November 14, 2023]
- Report 93: Pfizer’s ‘Post-Marketing Surveillance Report’ Reveals That Pfizer Manipulated Data and Wrongly Tabulated Adverse Events, Which Concealed Them. [November 28, 2023]
- Report 94: Pfizer Secretly Studied a Heart Damage Marker, Troponin I, in Five- to 15-Year-Olds, Following mRNA COVID Vaccination in 2021. [January 2, 2024]
- Report 95: mRNA COVID-19 Shots – ‘Vaccines’ or Gene Therapy Products? – Part 1 [February 19, 2024]
- Report 96: mRNA COVID-19 Shots – ‘Vaccines’ or Gene Therapy Products? – Part 2 [February 20, 2024]
- Report 97: Pfizer Obscured Myocarditis Safety Signal Specific to Young Men. FDA Took Five Months to Notice Pfizer’s Obfuscation. [March 25, 2024]
- Report 98: FDA Selected Its ‘Vaccines Advisory Committee’ – Not Its Gene Therapy Advisory Committee – to Recommend the COVID Injections for Emergency Use, to Hide the Fact that the Products Are Not Vaccines But Gene Therapies [April 11, 2024]
- Report 99: How the CDC Hides COVID Vaccine Deaths and Injuries [May 29, 2024]
- Report 100: Pathological Basis of CoVax Disease Cardiovascular Manifestations [July 11, 2024]
- Report 101: Gain-of-Function Research and Origin of SARS-CoV-2, the Virus That Causes COVID-19 [August 2, 2024]
- Report 102: Did Pfizer’s Product Pipeline Knowingly Plan for a Post-Vaccination Rise in Cancers? [November 11, 2024]


CLICK ABOVE FOR DETAILS https://www.amazon.com/DailyClout-Documents-Analysis-Volunteers-Reports-ebook/dp/B0BSK6LV5D/

CLICK ABOVE FOR DETAILS https://www.amazon.com/Pfizer-Papers-Pfizers-Against-Humanity/dp/1648210376/

Articles by Children’s Health Defense
https://childrenshealthdefense.org/defender/moderna-clinical-trial-documents-injuries/
https://childrenshealthdefense.org/defender/deaths-injuries-pfizer-vaccine-trial-document-dump/
https://childrenshealthdefense.org/defender/fda-moderna-spikevax-covid-vaccine-lawsuit/
https://childrenshealthdefense.org/defender/fda-pfizer-documents-vaccine-adverse-events/
https://childrenshealthdefense.org/defender/pfizer-hired-600-people-vaccine-injury-reports/
https://childrenshealthdefense.org/defender/fda-releases-pfizer-vaccine-documents/
https://childrenshealthdefense.org/defender/pfizer-fda-delay-release-covid-vaccine-safety-data/
https://childrenshealthdefense.org/defender/fda-eight-months-produce-pfizer-safety-data/



James Roguski
310-619-3055
JamesRoguski.substack.com/archive
ControlBloodSugarNaturally.com
I claim no copyright of any kind whatsoever, over any of my work, ever. Everyone is encouraged to copy any and all of it, in part, or in full, and use it for whatever purposes they wish. In fact, I would be delighted if someone were to copy this entire body of work. I encourage everyone to duplicate and mirror it in its entirety. I also encourage everyone to adapt and utilize the information in whatever manner they deem appropriate. No citation or other reference is requested or required. It would actually bring me great joy to see this information multiply exponentially and “go viral”.
All content is free to all readers.
All support is deeply appreciated.
-
AuthorPosts
- You must be logged in to reply to this topic.