Home › Forums › JAMES ROGUSKI › James Roguski Discover more from James Roguski Exposing MDM (Mis-Information, Dis-Information and Mal-Information) Over 70,000 subscribers Type your email… Continue reading Sign in Evidence of Harm The sheer volume of documented evidence of harm is absolutely astonishing.
- This topic is empty.
-
AuthorPosts
-
2025-01-02 at 19:19 #458982
 Nat QuinnKeymaster
Nat QuinnKeymasterEvidence of Harm
The sheer volume of documented evidence of harm is absolutely astonishing.
Jan 02, 2025
FOR COMPLETE DETAILS: NotSafeAndNotEffective.com

All of the EVIDENCE OF HARM in this article has been published in PubMed, or by the FDA, Pfizer or Moderna.
For those who have been “vaccinated,” please review the information in this article and ask yourself this very simple question:
If the information in this article had been made available to you before you were incentivized, coerced, convinced or tricked into receiving the COVID-19 “vaccines,” and you were given a true opportunity to understand the potential harm, would you have still allowed someone to inject a slow-acting, technologically advanced biological weapon into your arm?
People are still getting jabbed.
People are still suffering.
People are still being permanently disabled.
People are still dying.
It is NOT okay to ignore this information.
Help save someone’s life.
Share this information with everyone you possibly can.

Pfizer knew, even before they submitted their original application for an Emergency Use Authorization, that their mRNA “vaccine” did not work, but they kept it a secret.
And they wanted to keep it a secret for 75 years.
If you haven’t already done so, please CLICK HERE to read Chapter 7.

April 30, 2021
Pfizer and the FDA already knew that the Pfizer “vaccine” was associated with 1223 deaths and was causing hundreds of different diseases (“adverse events”).
They ignored the facts, claimed that their product was “safe and effective” and attempted to keep the truth hidden for 75 years.
Pfizer’s “Cumulative Analysis of Post-authorization Adverse Event Reports”
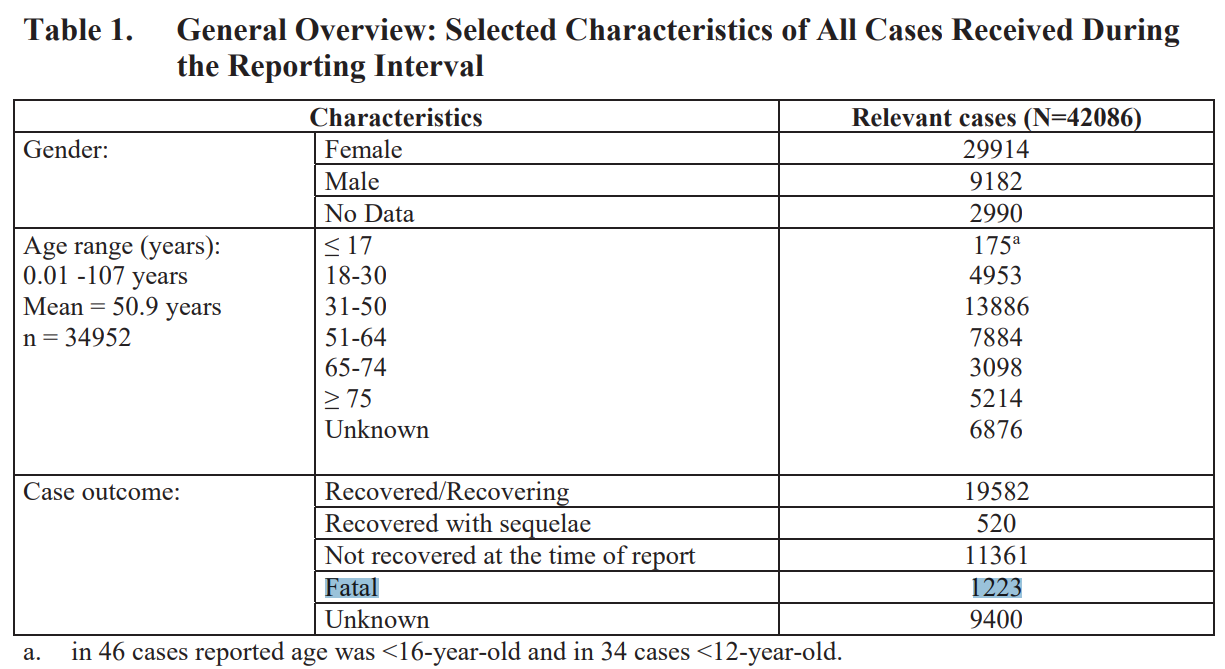
5. SUMMARY AND CONCLUSION
Review of the available data for this cumulative PM experience, confirms a favorable benefit: risk balance for BNT162b2.
https://phmpt.org/wp-content/uploads/2022/04/reissue_5.3.6-postmarketing-experience.pdf
The graphs below represent just some of the data in Pfizer’s report that shows women reported a much greater rate of adverse events than men.
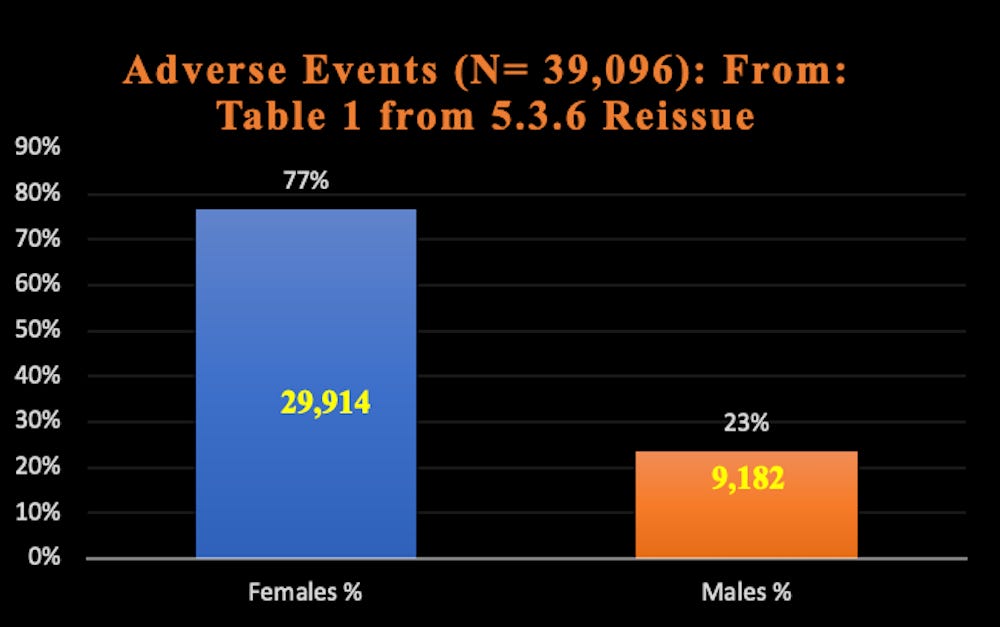
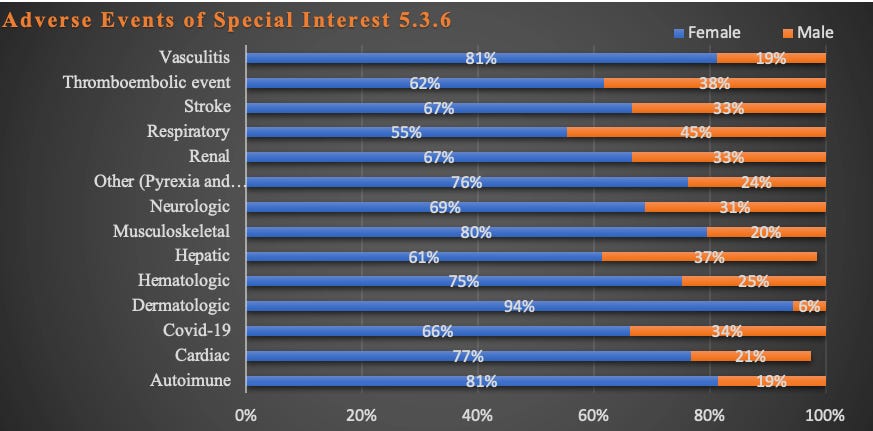
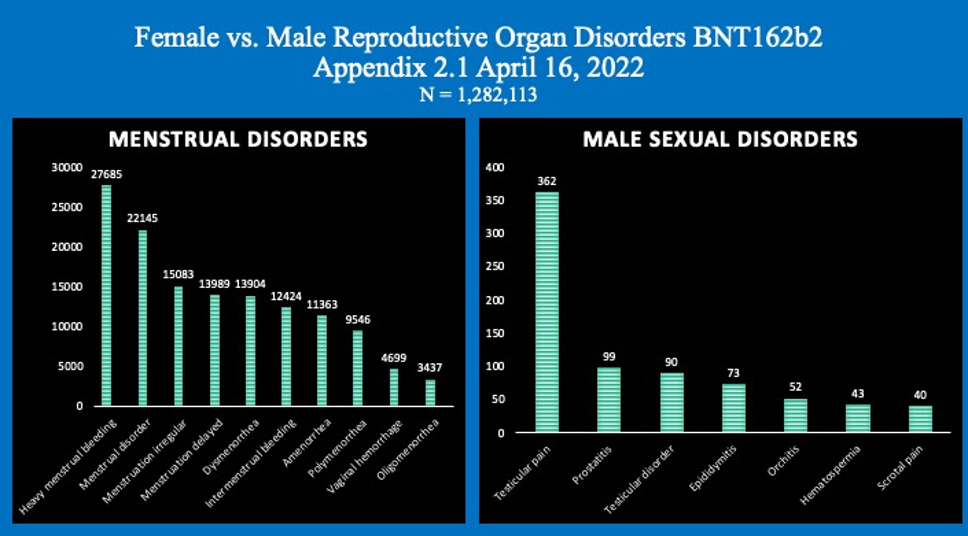
Graphs: Report 38: Women Have Two and a Half Times Higher Risk of Adverse Events Than Men. Risk to Female Reproductive Functions Is Higher Still [August 20, 2022]
APPENDIX 1. LIST OF ADVERSE EVENTS OF SPECIAL INTEREST.
The number of different manifestations of adverse events was/is enormous.









The 9 pages above merely list the names of diseases or “adverse events” that were KNOWN to Pfizer by April 30, 2021.
The 393-page document below details the extent of the suffering that Pfizer knew was caused by their “vaccine.”
The extent of the harm that Pfizer’s product has caused is astonishing.
https://www.globalresearch.ca/wp-content/uploads/2023/05/pfizer-report.pdf

Biodistribution study
The ingredients in the injections do NOT remain in the shoulder muscle.
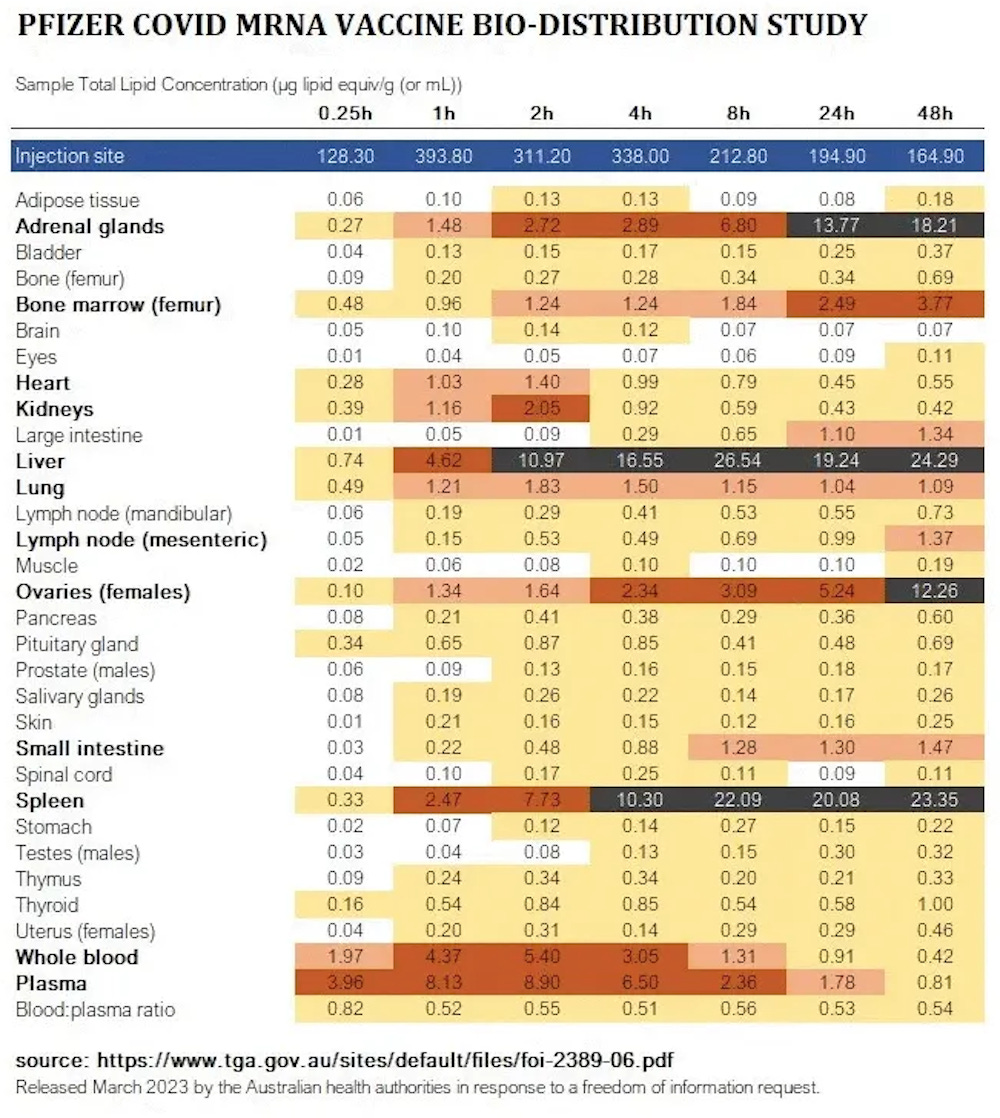
https://www.docdroid.net/xq0Z8B0/pfizer-report-japanese-government-pdf#page=17

July 21, 2022
German authorities’ confession of side effects puts a bomb under vaccination policy
The German Ministry of Health officially admitted on Wednesday that 1 in 5,000 corona jabs leads to hospitalization, permanent disability or death.
In the thread below the tweet the ministry makes a correction, that it is not about 1 in 5,000 people but about 1 in 5,000 injections. Someone who gets four injections has a chance of 1:1250 of a serious side effect.

August 3, 2022
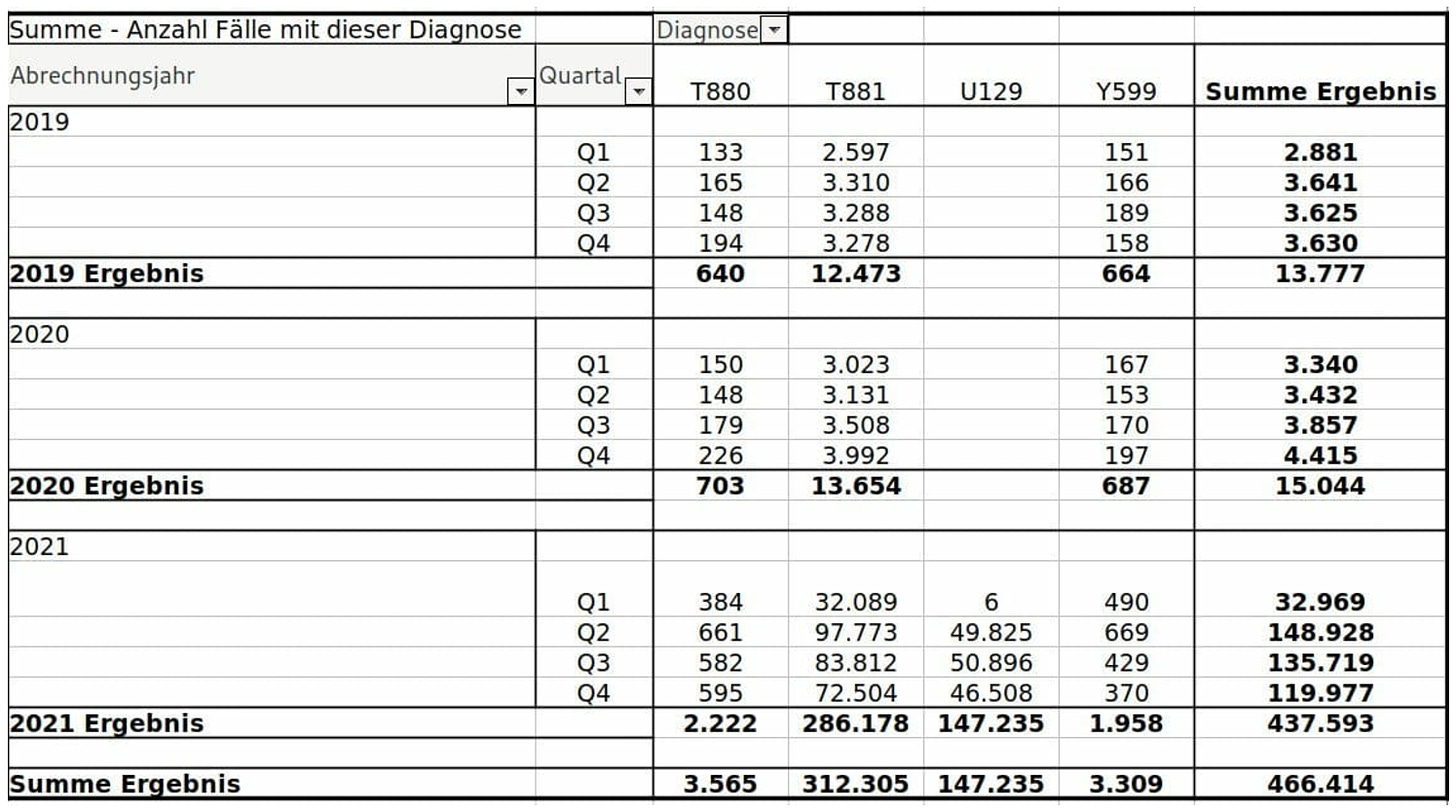
Germany’s Largest Health Insurer Reveals 1 in 25 Clients Underwent Medical Treatment in 2021 for Covid ‘Vaccine’ Side Effects
Based on the figures from Techniker Krankenkasse, as many as 1 in 500 covid vaccine injections are expected to cause serious side effects.
The data shows that in 2021 the Techniker Krankenkasse had to reimburse 147,235 medical treatments for code U12.9 – Adverse reactions to the use of covid vaccines, unspecified – alone. All codes listed below are serious side effects requiring a doctor’s treatment.
In 2019, among the 11 million insured, 13,777 medical treatments were required due to vaccine side effects. In 2020 there were 15,044. In 2021, the number shot up to 437,593, an increase of more than 3,000 percent.
As many as 1 in 500 injections is expected to cause serious side effects.
Serious side effects include (facial) paralysis, persistent pain, nerve problems, severe skin reactions, heart attacks, strokes, heart muscle inflammation, permanent disability, and death.
- T881 – Complications after vaccination (immunization), not classified elsewhere, including rash after vaccination
- T88.0 – Post-vaccination infection (immunization), including post-vaccination sepsis (immunization),
- U12.9 – Adverse reactions to the use of COVID-19 vaccines, unspecified
- Y59.9 – Adverse complications due to vaccines or biologically active substances.

March 12, 2024
PFIZER NON-INTERVENTIONAL INTERIM STUDY REPORT 5
The overall goal of the study is to determine whether an increased risk of prespecified adverse events of special interest (AESIs) exists following the administration of at least one dose of the Pfizer- BioNTech COVID-19 vaccine. This non-interventional study is designated as a Post-Authorization Safety Study (PASS) and is a commitment to the EMA and a Postmarketing Requirement to the Food and Drug Administration (FDA).
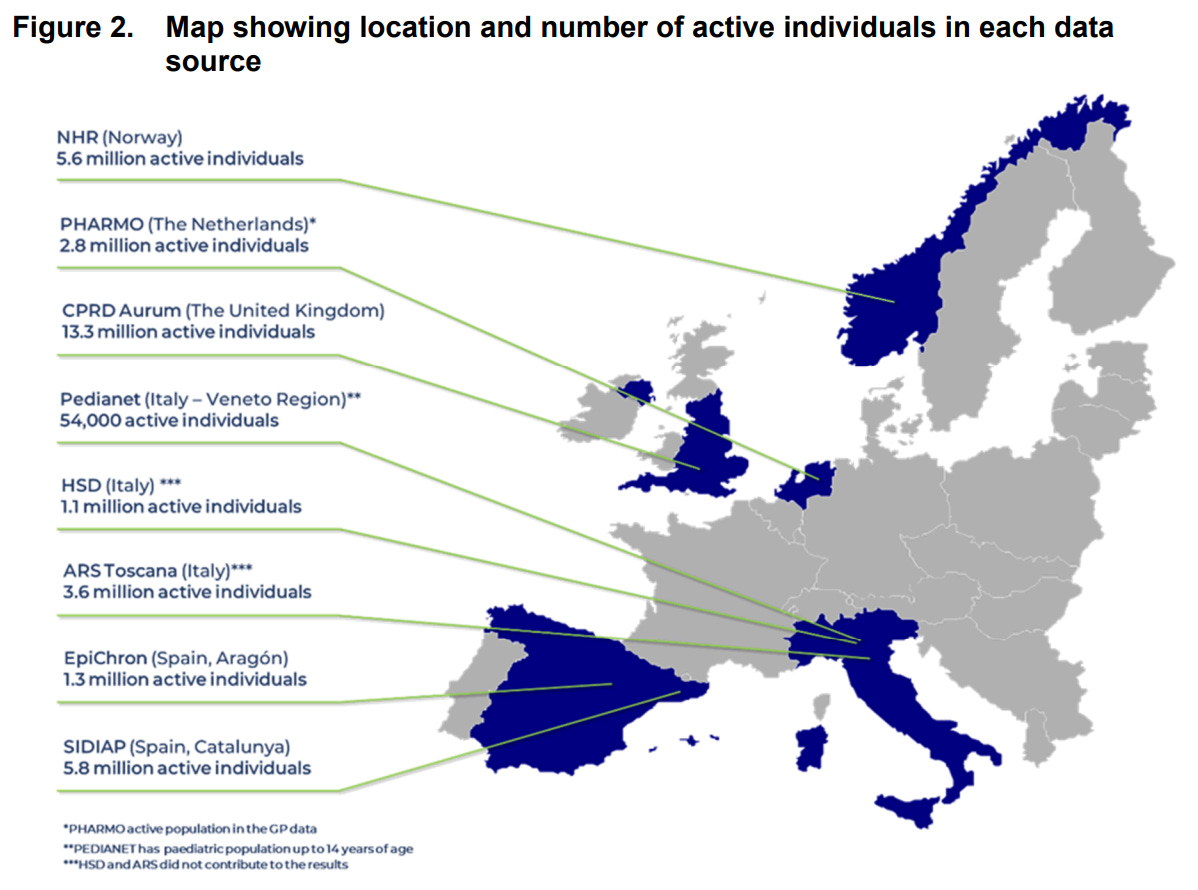
The graphs below specify results collected through CPRD (Clinical Practice Research Datalink) Aurum (UK), the largest data set with over 13 million active individuals.
Figure 6. Cumulative incidence of acute cardiovascular injury among individuals who received at least one dose of Pfizer-BioNTech COVID-19 vaccine and matched unvaccinated individuals by data source (risk window: 365 days after dose 1)
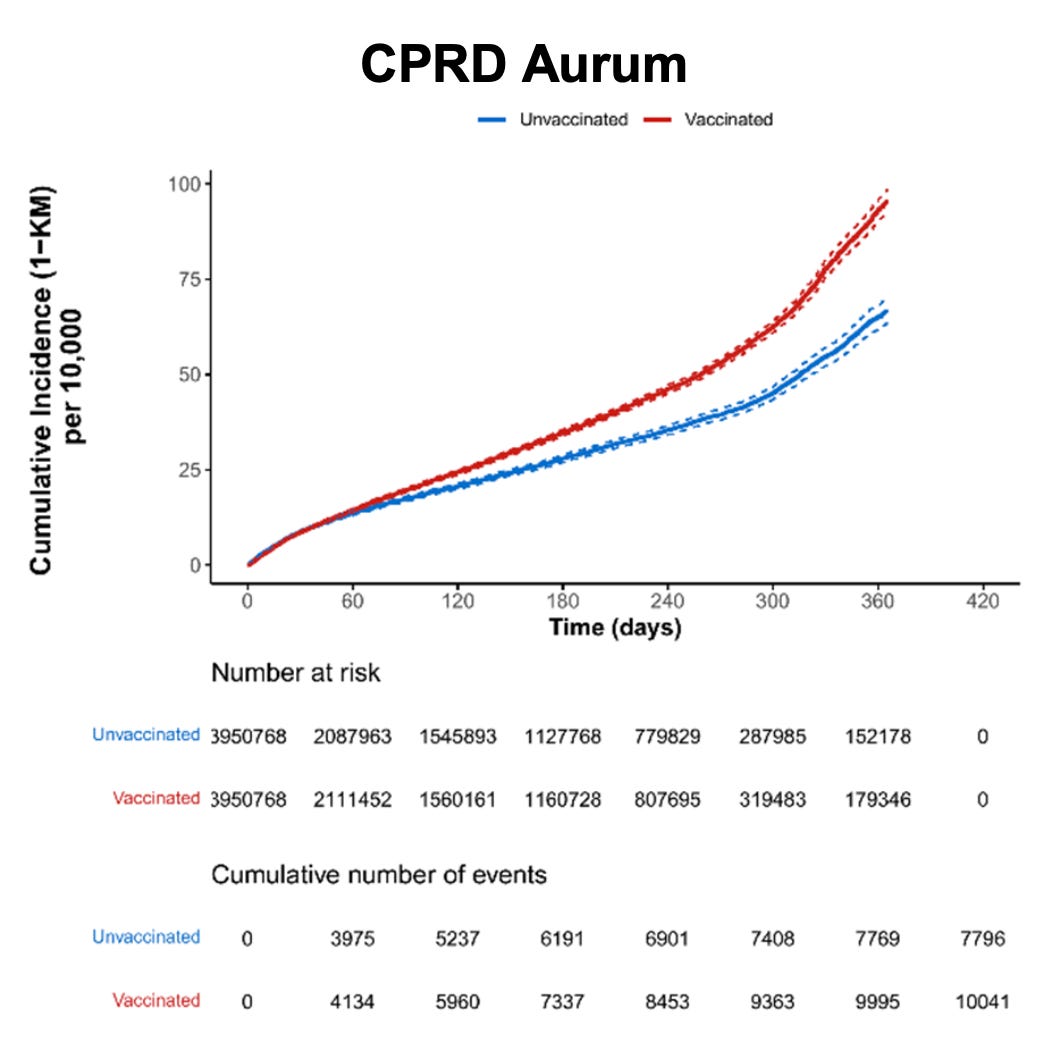
Figure 8. Cumulative incidence of arrhythmia among individuals who received at least one dose of Pfizer-BioNTech COVID-19 vaccine and matched unvaccinated individuals by data source (risk window: 365 days after dose 1)
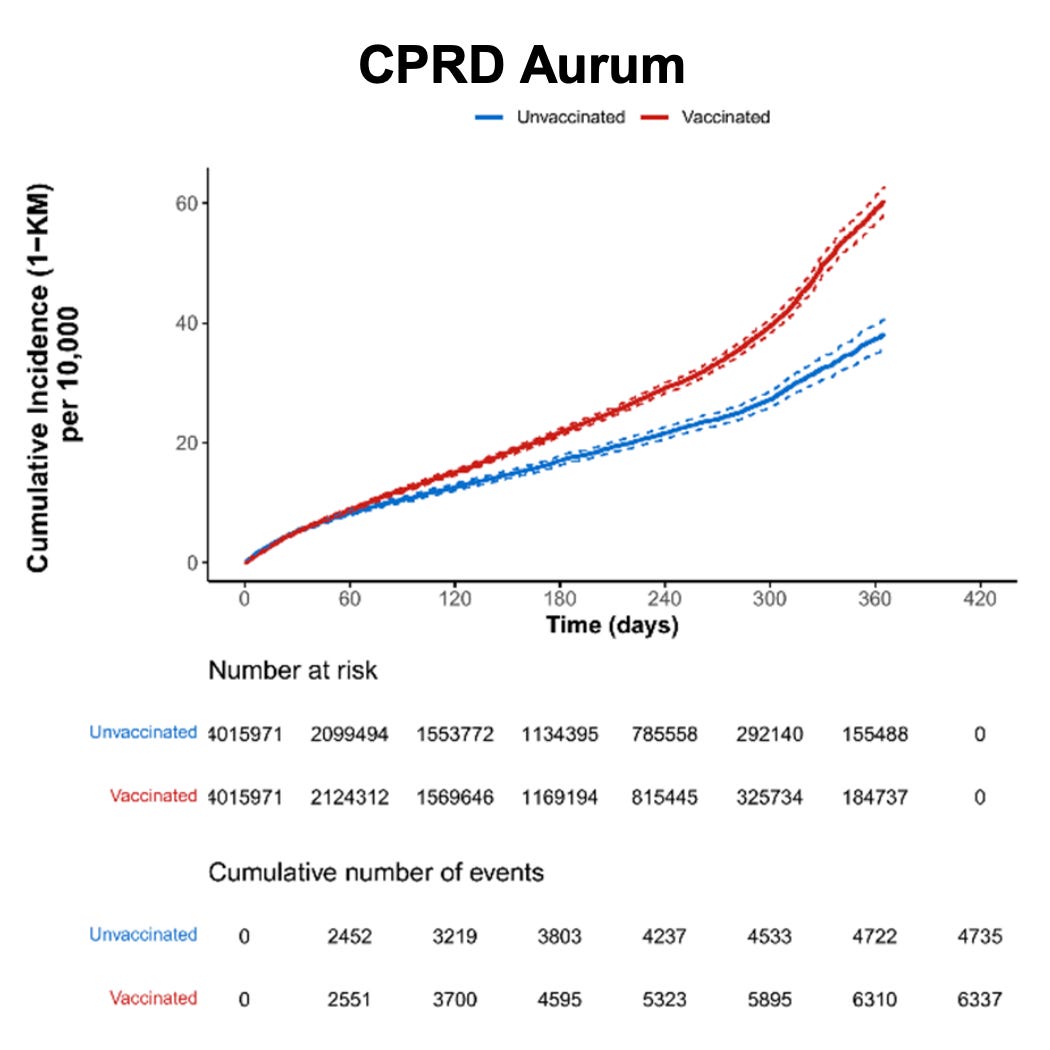
Figure 10. Cumulative incidence of heart failure among individuals who received at least one dose of Pfizer-BioNTech COVID-19 vaccine and matched unvaccinated individuals by data source (risk window: 365 days after dose 1)
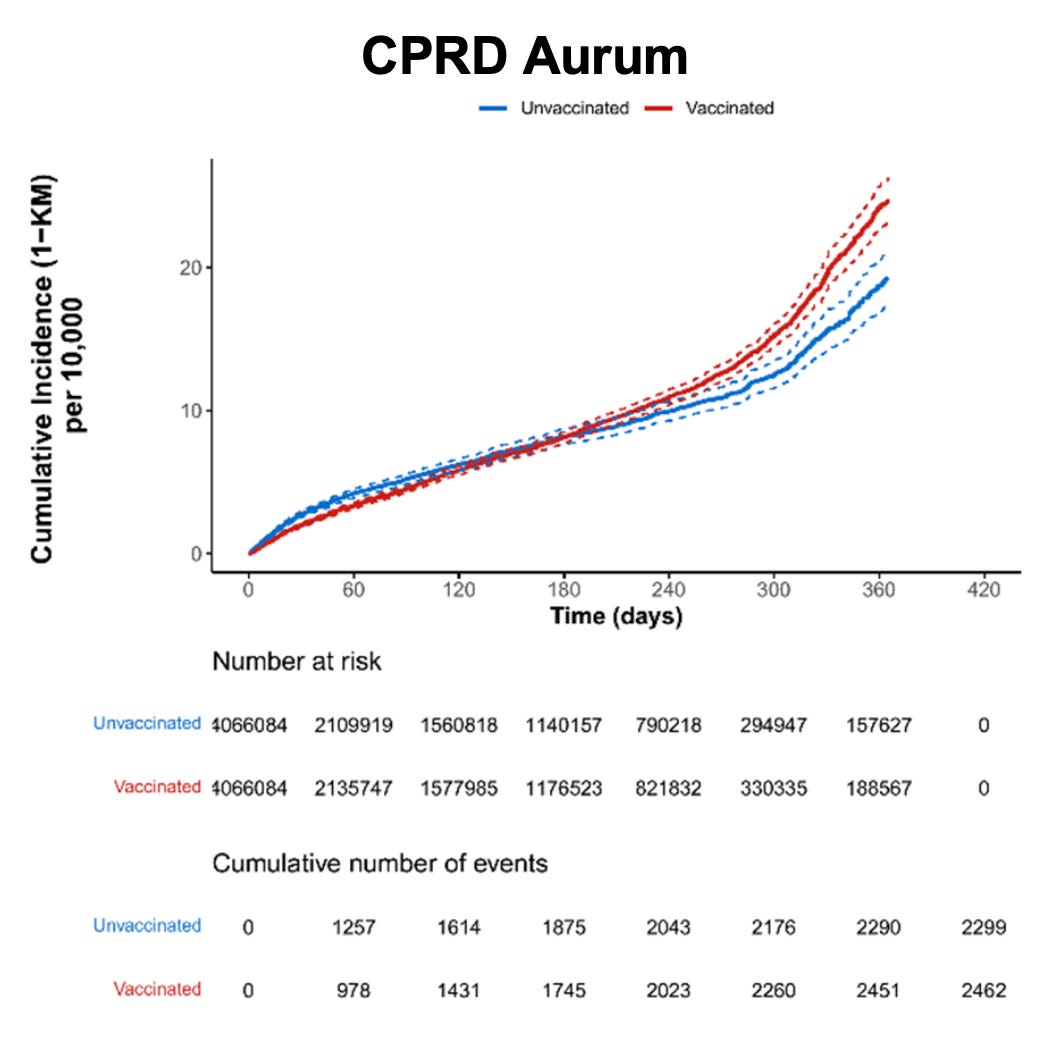
Figure 14. Cumulative incidence of coronary artery disease among individuals who received at least one dose of Pfizer-BioNTech COVID-19 vaccine and matched unvaccinated individuals by data source (risk window: 365 days after dose 1)
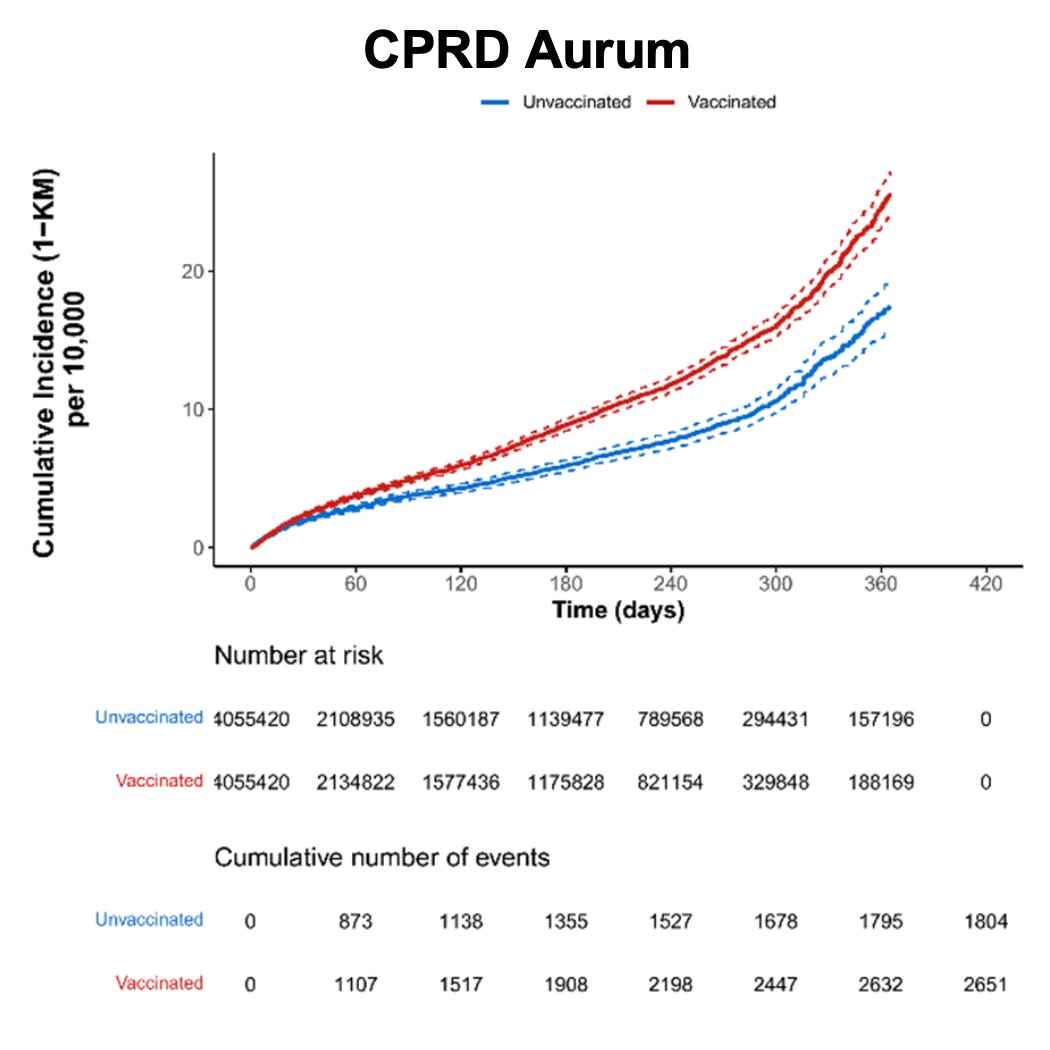
Figure 24. Cumulative incidence of secondary amenorrhoea within 183 days after start of follow-up among individuals who received at least one dose of Pfizer-BioNTech COVID-19 vaccine and matched unvaccinated individuals by data source
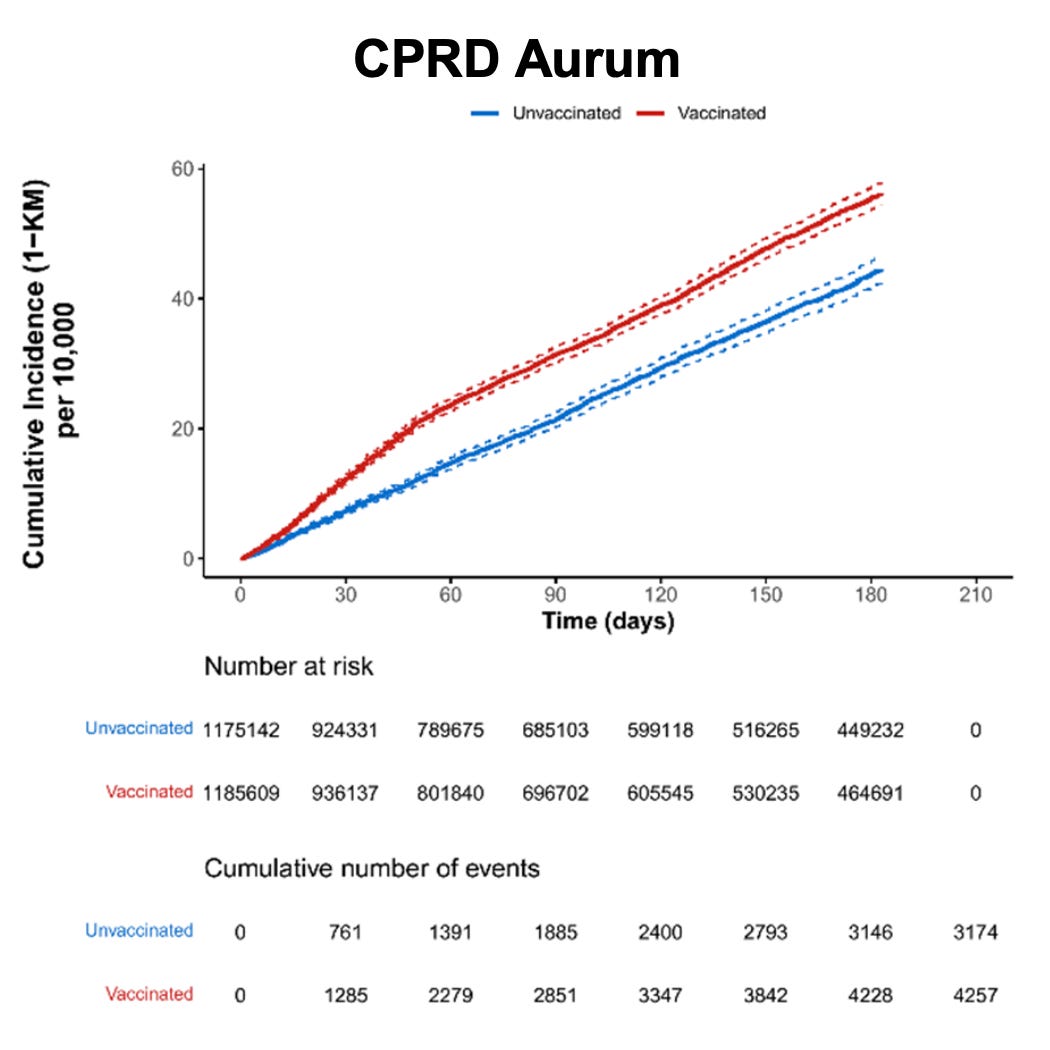
From Table 16, note the following INCREASED RATES OF ADVERSE EVENTS (for participants via CPRD Aurum):
- Acute aseptic arthritis: 1.23
- Diabetes mellitus type 1: 1.2
- Idiopathic Thrombocytopenia: 1.4
- Acute cardiovascular injury including microangiopathy: 1.23
- Arrhythmia: 1.27
- Stress cardiomyopathy: 1.3
- Coronary artery disease: 1.4
- Myocarditis (7 days): 9.70
- Myocarditis (14 days): 1.74
- Myocarditis (21 days): 2.3
- Pericarditis (7 days): 1.1
- Pericarditis (14 days): 1.36
- Pericarditis (21 days): 1.40
- Myocarditis or pericarditis (7 days): 1.8
- Myocarditis or pericarditis (14 days): 1.49
- Myocarditis or pericarditis (21 days): 1.68
- Secondary amenorrhoea: 1.25
- Anaphylaxis: 1.4
- Multisystem inflammatory syndrome: 3.36
- Subacute thyroiditis: 2.98
https://dailysceptic.org/wp-content/uploads/2024/12/06-C4591021-interim5-report-body.pdf (pages 118-126)
DOCUMENTS:
https://dailysceptic.org/wp-content/uploads/2024/12/06-C4591021-interim5-report-body.pdf


PubMed Articles
Below is just a small sampling of the thousands of articles that have been published in peer-reviewed journals and may be accessed by all via the PubMed database.
- 30587973 Potential adverse effects of nanoparticles on the reproductive system
- 34033367 Coronavirus (COVID-19) Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) (Archived)
- 34365148 Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review
- 34432976 Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting
- 34449596 Synthetic mRNAs; Their Analogue Caps and Contribution to Disease
- 34477808 Surveillance for Adverse Events After COVID-19 mRNA Vaccination
- 34719776 Spectrum of neurological complications following COVID-19 vaccination
- 34724709 SARS-CoV-2 spike-dependent platelet activation in COVID-19 vaccine-induced thrombocytopenia
- 34908713 Dangers of mRNA vaccines
- 34935921 Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination
- 35186864 Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States
- 35202800 Thrombosis and thrombocytopenia in COVID-19 and after COVID-19 vaccination
- 35263195 Evaluation of Adverse Effects in Nursing Mothers and Their Infants After COVID-19 mRNA Vaccination
- 35369340 Cardiovascular Complications of COVID-19 Vaccines
- 35436552 Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs
- 35455237 The Side Effects and Adverse Clinical Cases Reported after COVID-19 Immunization
- 35484304 Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave
- 35537987 Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis
- 35579205 Amyloidogenesis of SARS-CoV-2 Spike Protein
- 35746474 What Should We Do after the COVID-19 Vaccination? Vaccine-Associated Diseases and Precautionary Measures against Adverse Reactions
- 35753869 COVID-19, vaccines and deficiency of ACE2 and other angiotensinases. Closing the loop on the “Spike effect”
- 35765616 Safety and Adverse Events Related to COVID-19 mRNA Vaccines; a Systematic Review
- 35779962 Vaccine-induced immune thrombotic thrombocytopenia after COVID-19 vaccination: Description of a series of 39 cases in Brazil
- 35805941 Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series
- 35885461 Current Evidence in SARS-CoV-2 mRNA Vaccines and Post-Vaccination Adverse Reports: Knowns and Unknowns
- 35971401 Catecholamines Are the Key Trigger of COVID-19 mRNA Vaccine-Induced Myocarditis: A Compelling Hypothesis Supported by Epidemiological, Anatomopathological, Molecular, and Physiological Findings
- 35995416 Factors related to the serious adverse events in patients visiting the emergency department after ChAdOx1 and mRNA COVID-19 vaccination
- 36016112 Cross-Sectional Survey on BNT162b2 mRNA COVID-19 Vaccine Serious Adverse Events in Children 5 to 11 Years of Age: A Monocentric Experience
- *36055877 Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults
- 36074641 Anti-spike T-cell and Antibody Responses to SARS-CoV-2 mRNA Vaccines in Patients with Hematologic Malignancies
- 36109582 Reports of acute adverse events in mRNA COVID-19 vaccine recipients after the first and second doses in Japan
- 36114089 [COMMENT] The spike hypothesis in vaccine-induced adverse effects: questions and answers
- 36142792 Understanding the Pharmacology of COVID-19 mRNA Vaccines: Playing Dice with the Spike?
- 36274082 COVID-19 mRNA Vaccines: A Retrospective Observational Pharmacovigilance Study
- 36371366 A post-marketing safety assessment of COVID-19 mRNA vaccination for serious adverse outcomes using administrative claims data linked with vaccination registry in a city of Japan
- 36419624 Serious adverse reaction associated with the COVID-19 vaccines of BNT162b2, Ad26.COV2.S, and mRNA-1273: Gaining insight through the VAERS
- 36445631 Neurological Complications Following COVID-19 Vaccination
- 36514568 Reactogenicity of COVID-19 Vaccines in Patients With a History of COVID-19 Infection: A Survey Conducted in Pakistan
- 36555121 SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects
- 36597886 Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis
- *36605446 Evidence of exhausted lymphocytes after the third anti-SARS-CoV-2 vaccine dose in cancer patients
- *36961579 Safety of COVID-19 vaccines in pregnancy: a VAERS based analysis
- 36988252 Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia
- 36997290 Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine
- 37067070 Temporal association between COVID-19 vaccination and Raynaud’s phenomenon: A case series
- 37094803 Neurological Considerations with COVID-19 Vaccinations
- 37121802 Serious adverse events following mRNA vaccination in randomized trials in adults
- 37272559 A Nationwide Survey of mRNA COVID-19 Vaccinee’s Experiences on Adverse Events and Its Associated Factors
- 37360861 Risk of carditis after three doses of vaccination with mRNA (BNT162b2) or inactivated (CoronaVac) covid-19 vaccination: a self-controlled cases series and a case-control study
- 37445690 mRNA: Vaccine or Gene Therapy? The Safety Regulatory Issues
- 37531110 Comparative Risks of Potential Adverse Events Following COVID-19 mRNA Vaccination Among Older US Adults
- *37626783 ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA
- 37192595 COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVID Vac-syndrome”): Similarities and differences
- 37710966 Autoimmune inflammatory reactions triggered by the COVID-19 genetic vaccines in terminally differentiated tissues
- 37732332 Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases
- 37811764 An analysis of reported cases of hemophagocytic lymphohistiocytosis (HLH) after COVID-19 vaccination
- 37833825 Batch-dependent safety of the BNT162b2 mRNA COVID-19 vaccine
- 38101158 Gene-based COVID-19 vaccines: Australian perspectives in a corporate and global context
- *38221509 Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis
- 38293564 Determinants of COVID-19 vaccine-induced myocarditis
- *38350768 COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals
- 38383356 Comparative efficacy and safety of COVID-19 vaccines in phase III trials: a network meta-analysis
- 38390323 The mRNA-LNP vaccines – the good, the bad and the ugly?
- 38407875 Adverse Events After XBB.1.5-Containing COVID-19 mRNA Vaccines
- 38408769 Covid-19: Two rare vaccine side effects detected in large global study
- 38413637 Exploring the reported adverse effects of COVID-19 vaccines among vaccinated Arab populations: a multi-national survey study
- 38442719 Adverse Events Following COVID-19 Vaccination in Adolescents: Insights From Pharmacovigilance Study of VigiBase
- 38500575 Global Safety Assessment of Adverse Events of Special Interest Following 2 Years of Use and 772 Million Administered Doses of mRNA-1273
- 38543142 Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System
- 38834668 Psychiatric adverse events following COVID-19 vaccination: a population-based cohort study in Seoul, South Korea
- 38864106 Incidence and management of the main serious adverse events reported after COVID-19 vaccination
- 38926432 The direct effect of SARS-CoV-2 virus vaccination on human ovarian granulosa cells explains menstrual irregularities
- 38937903 Batch-dependent safety of COVID-19 vaccines in the Czech Republic and comparison with data from Denmark
- 38942751 Myocarditis associated with COVID-19 vaccination
- 39103148 SARS-CoV-2 mRNA vaccine-related myocarditis and pericarditis: An analysis of the Japanese Adverse Drug Event Report database
- 39171563 The systemic capillary leak syndrome following COVID-19 vaccine
- 39202624 Reports of Batch-Dependent Suspected Adverse Events of the BNT162b2 mRNA COVID-19 Vaccine: Comparison of Results from Denmark and Sweden
- *39312602 Evidence Review of the Adverse Effects of COVID-19 Vaccination and Intramuscular Vaccine Administration
- 39600629 Deaths Related to New-Onset Seizures After Vaccination

- Conclusion 7-1: The evidence establishes a causal relationship between the BNT162b2 vaccine and myocarditis. (Pfizer)
- Conclusion 7-2: The evidence establishes a causal relationship between the mRNA-1273 vaccine and myocarditis. (Moderna)
- Conclusion 10-1: The evidence establishes a causal relationship between vaccine administration and subacromial/subdeltoid bursitis caused by direct injection into the bursa.
- Conclusion 10-2: The evidence establishes a causal relationship between vaccine administration and acute rotator cuff or acute biceps tendinopathy caused by direct injection into or adjacent to the tendon.
- Conclusion 10-6: The evidence establishes a causal relationship between vaccine administration and bone injury caused by direct injection into or adjacent to the bone.
- Conclusion 10-7: The evidence establishes a causal relationship between vaccine administration and axillary or radial nerve injury caused by direct injection into or adjacent to the nerve.
Evidence Review Of The Adverse Effects Of Covid 19 Vaccination And Intramuscular Vaccine Administration4.31MB ∙ PDF filehttps://www.ncbi.nlm.nih.gov/books/NBK607376/pdf/Bookshelf_NBK607376.pdf

The article below was first published by Global Research on January 21, 2022. Since the publication of this article, the number of studies has increased dramatically. The evidence is overwhelming.

https://www.globalresearch.ca/covid-19-vaccines-scientific-proof-lethality/5767711

3900+ Case Studies

Informed consent requires information.
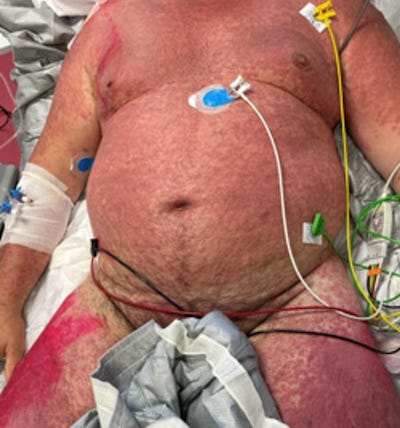
Below are just a small sample of the many PUBLISHED case studies of harm that has been caused by the COVID-19 bioweapons.
If people were shown the images below prior to receiving the jabs, do you think that many of them would have refused to comply?

34131967 Psoriasis flare-up associated with second dose of Pfizer-BioNTech BNT16B2b2 COVID-19 mRNA vaccine
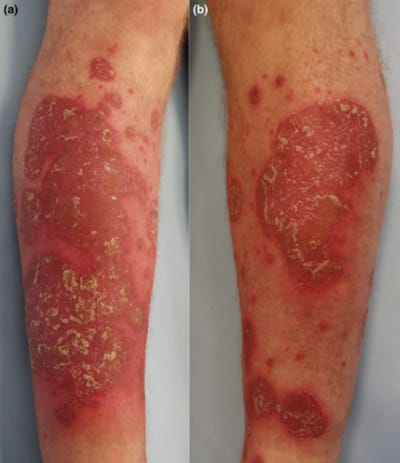
34162525 “COVID Toes” After mRNA COVID-19 Vaccines
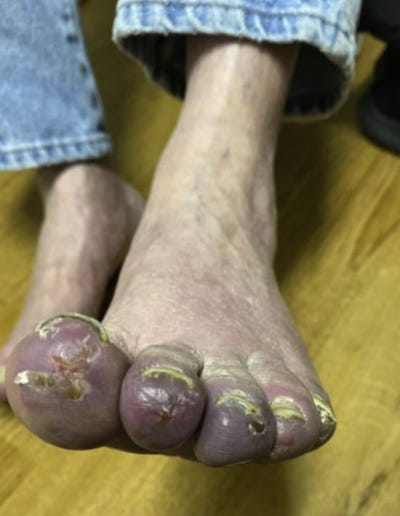
34169578 The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: is the second dose therefore contraindicated?
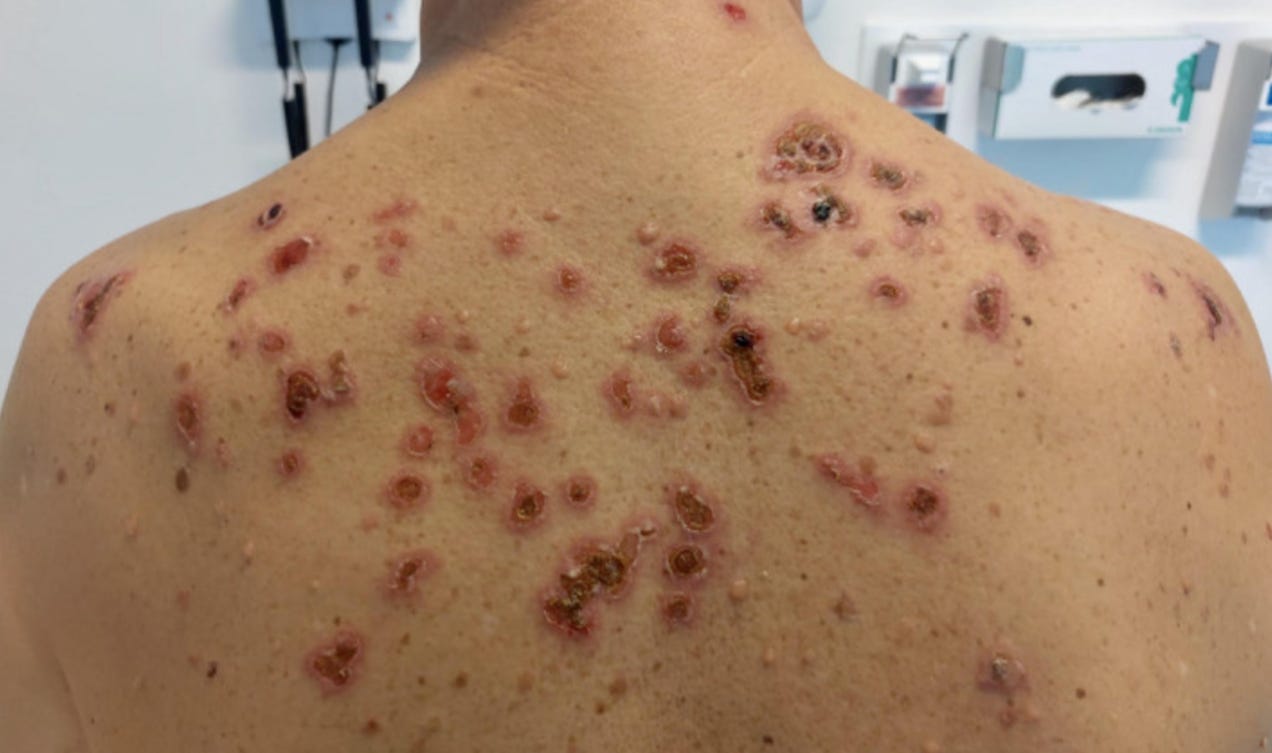
34236711 Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature

34294590 Bullous drug eruption after second dose of mRNA-1273 (Moderna) COVID-19 vaccine: Case report
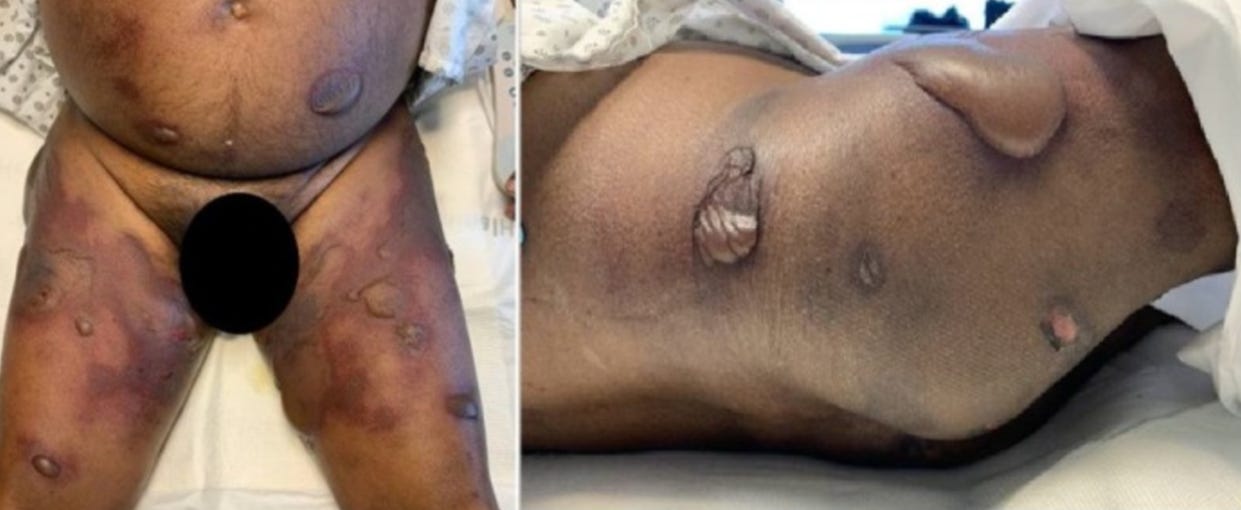
34379821 Pityriasis rubra pilaris-like eruption following administration of the BNT163b2 (Pfizer-BioNTech) mRNA COVID-19 vaccine
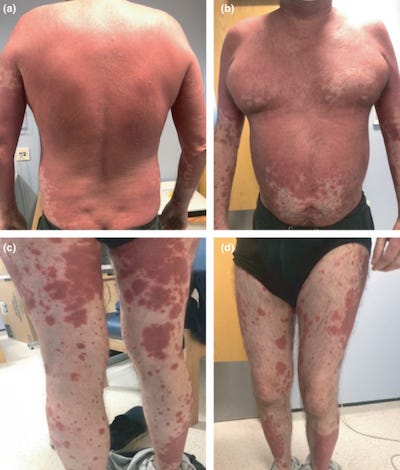
34423142 Moderna COVID-19 vaccine induced skin rash
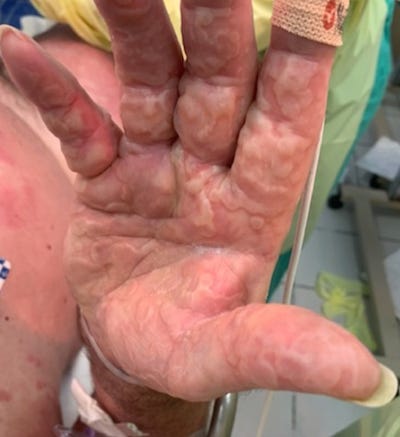
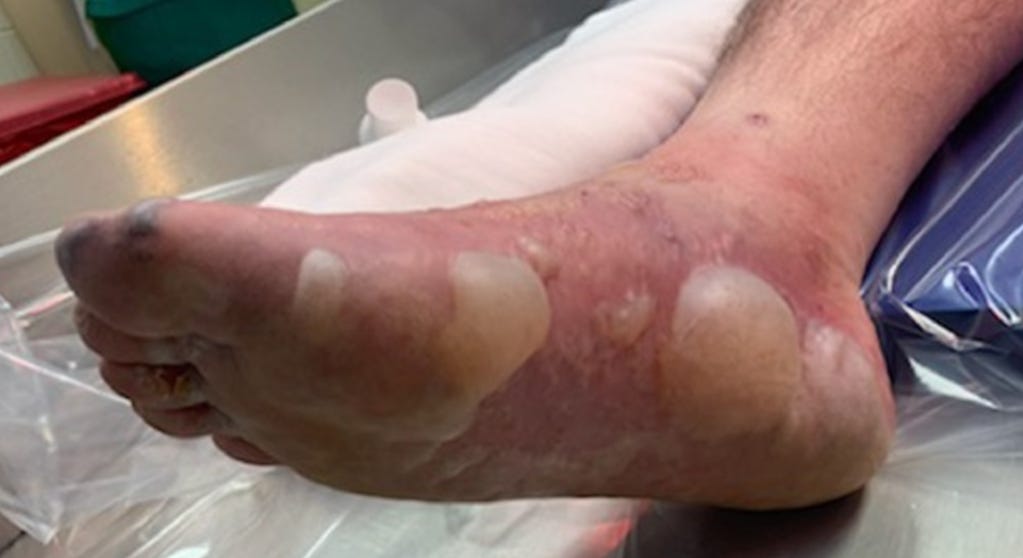
34473839 Atypical erythema multiforme related to BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine
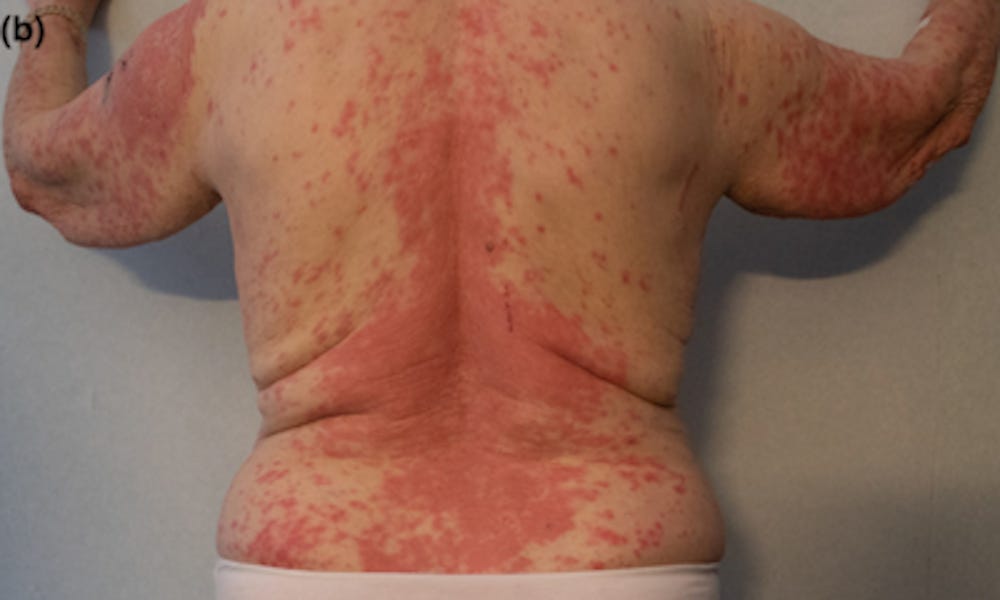
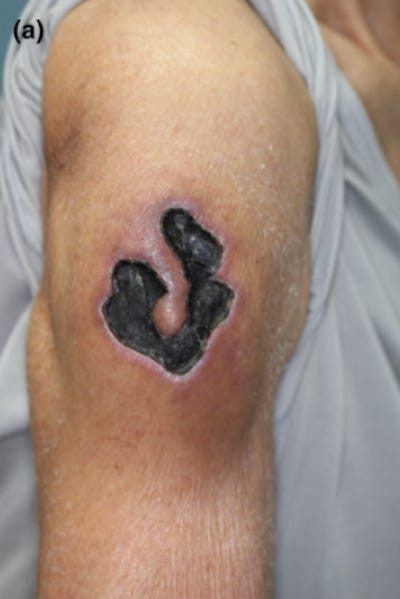
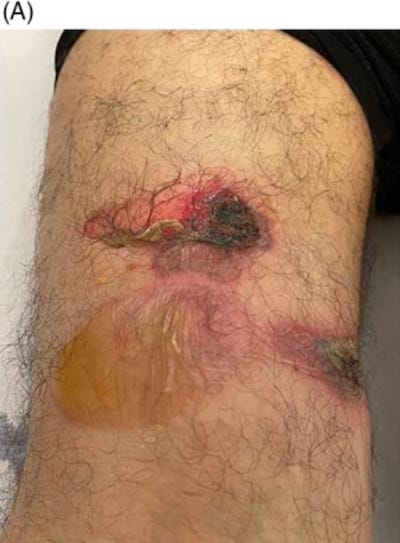
34545609 Skin ulcer at the injection site of BNT162b2 mRNA COVID-19 vaccine
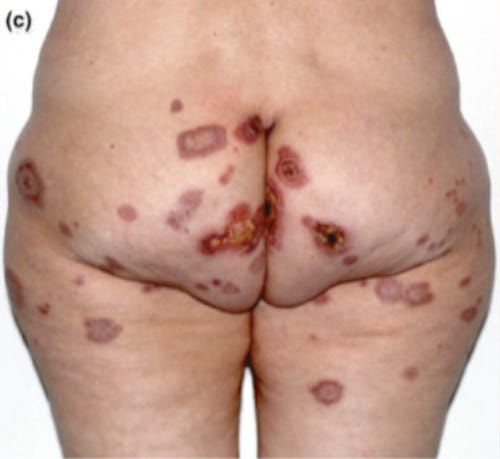
34617317 Abrupt onset of Sweet syndrome, pityriasis rubra pilaris, pityriasis lichenoides et varioliformis acuta and erythema multiforme: unravelling a possible common trigger, the COVID-19 vaccine
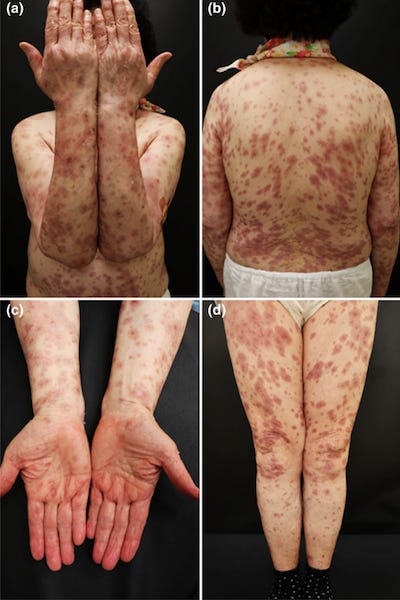
34661942 Generalized erythema multiforme-like skin rash following the first dose of COVID-19 vaccine (Pfizer-BioNTech)
34750923 “Covid arm”: Abnormal side effect after Moderna COVID-19 vaccine
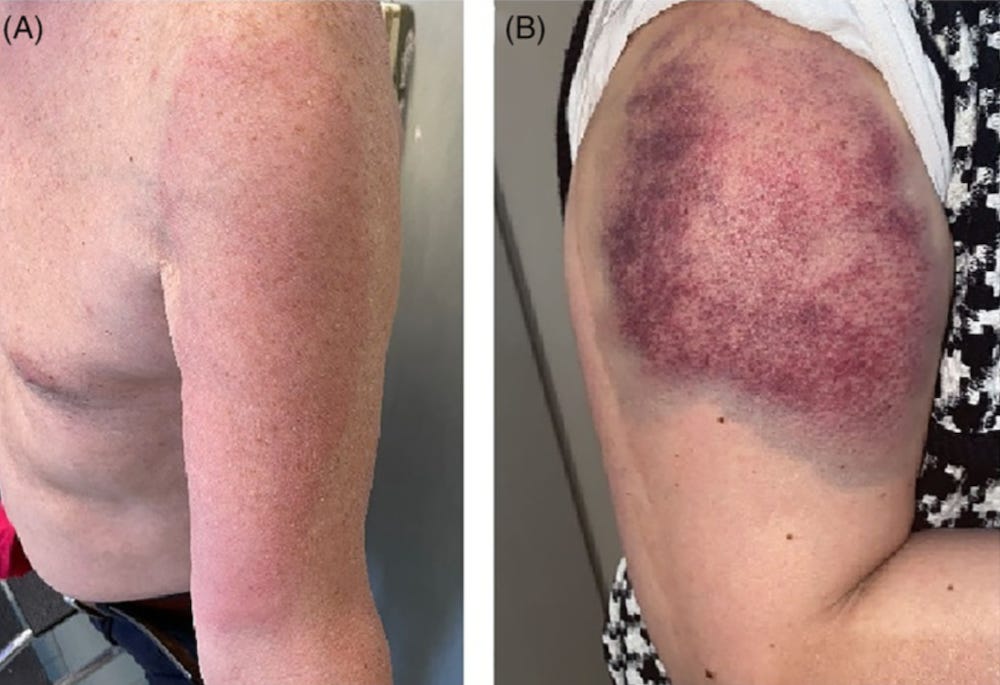
34786801 Bullous pemphigoid triggered by COVID-19 vaccine: Rapid resolution with corticosteroid therap
34835143 Sweet Syndrome Following SARS-CoV2 Vaccination
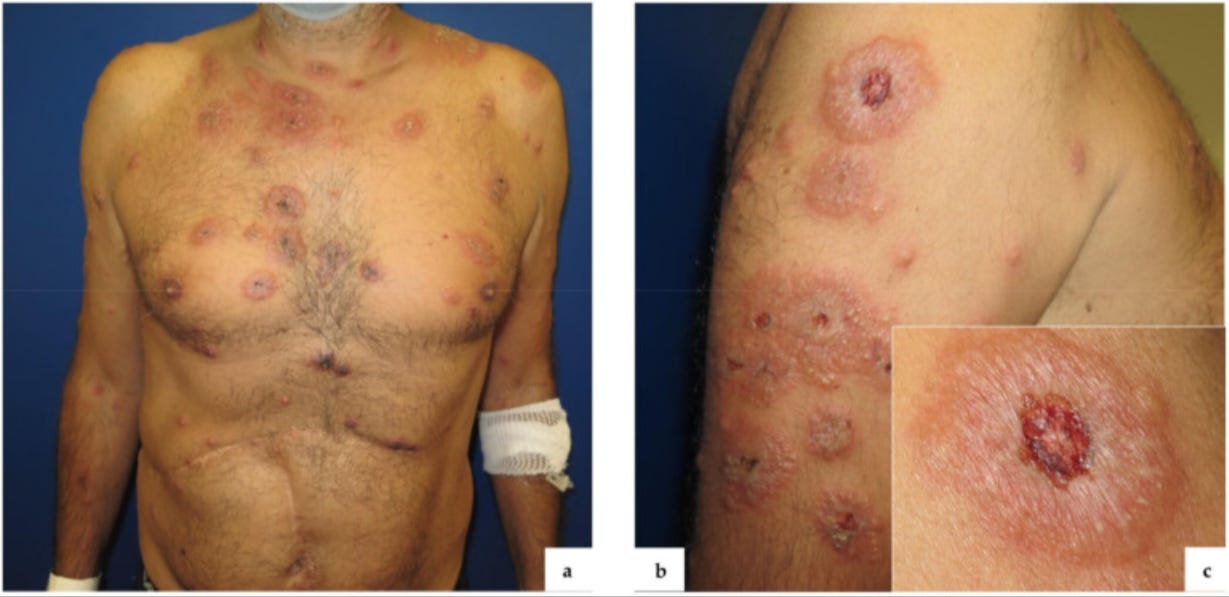
34837354 Cutaneous reactions to COVID-19 vaccine at the dermatology primary care
35062748 Pyoderma Gangrenosum Induced by BNT162b2 COVID-19 Vaccine in a Healthy Adult
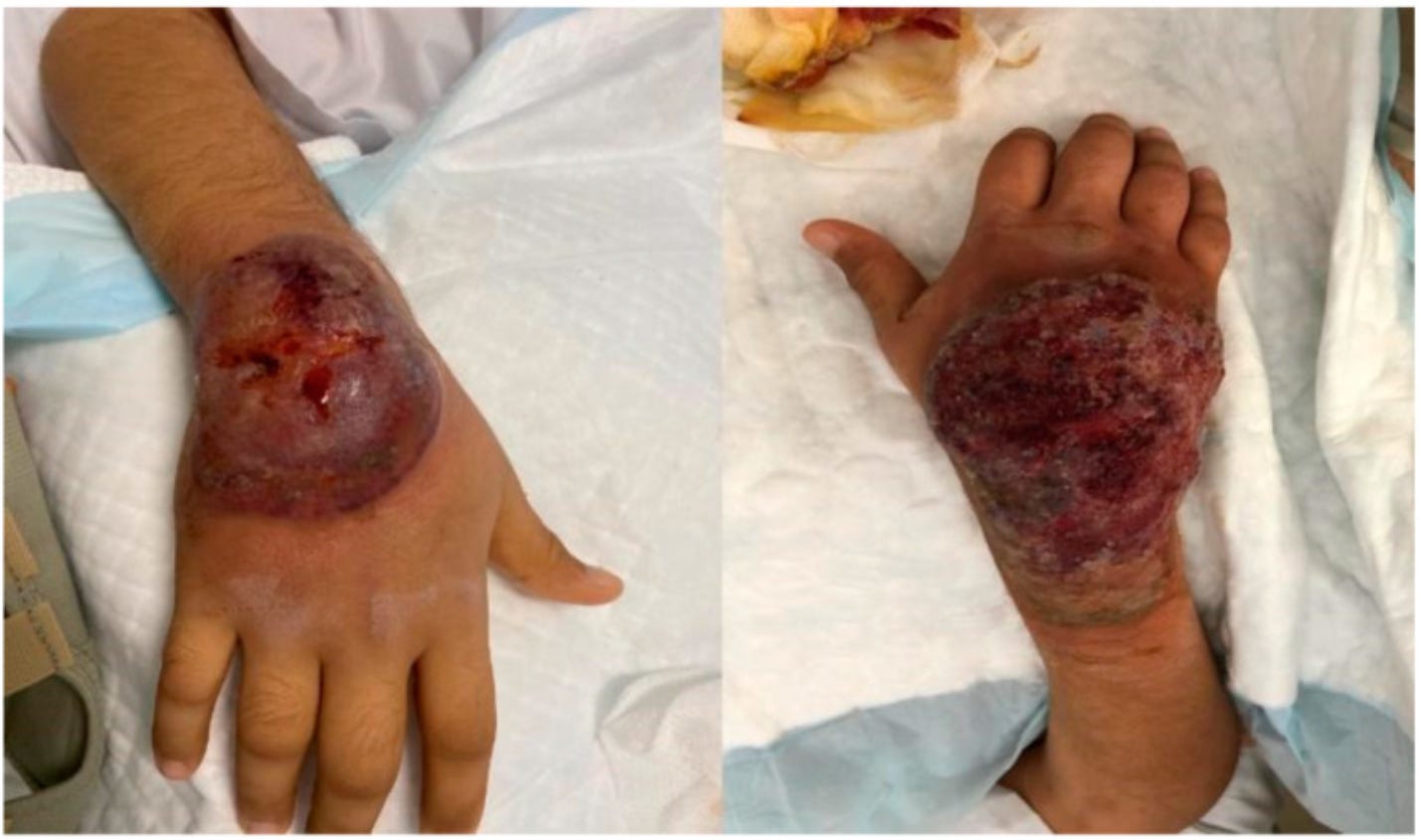
35191544 Exacerbation of systemic lupus erythematosus after receiving mRNA-1273-based coronavirus disease 2019 vaccine
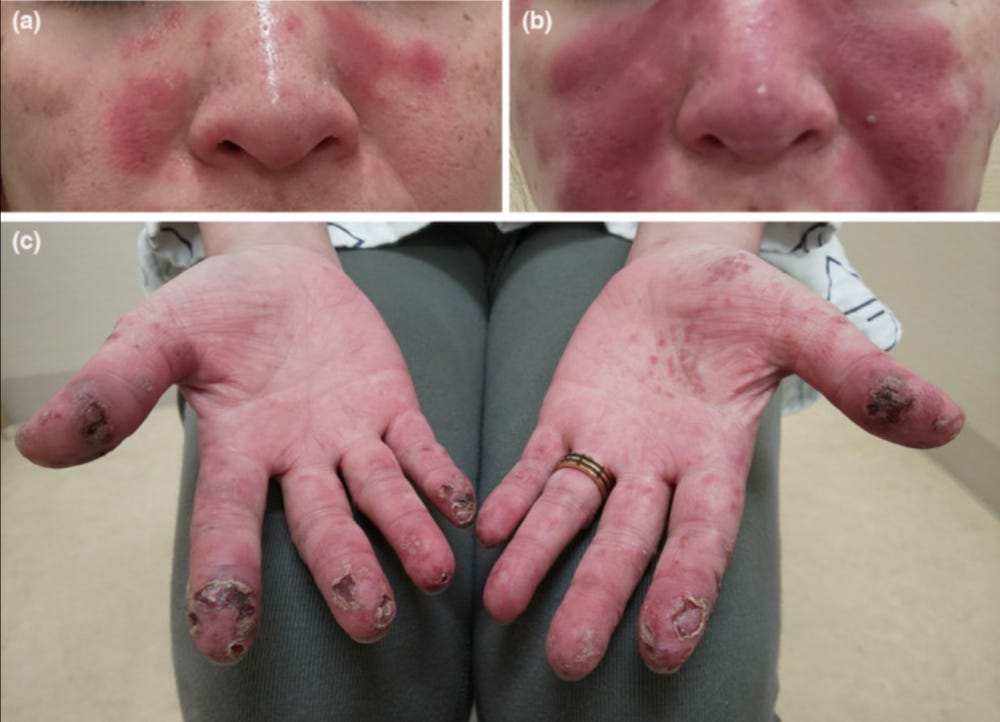
35224780 Generalized pustular psoriasis flare in a patient affected by plaque psoriasis after BNT162b2 mRNA COVID-19 vaccine, successfully treated with risankizumab
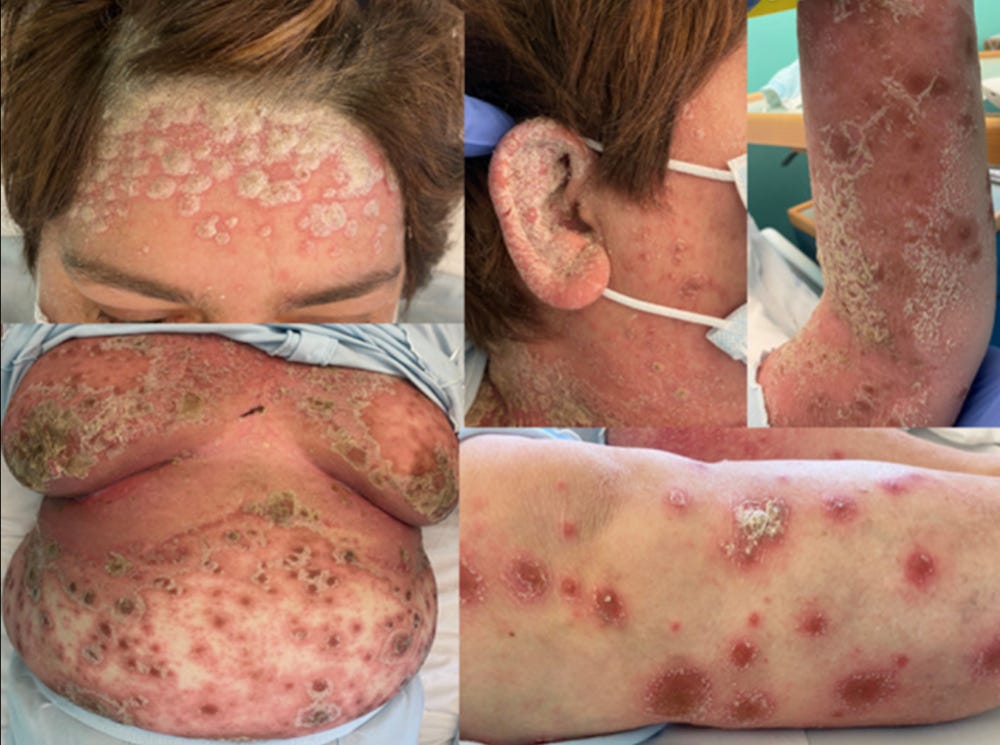
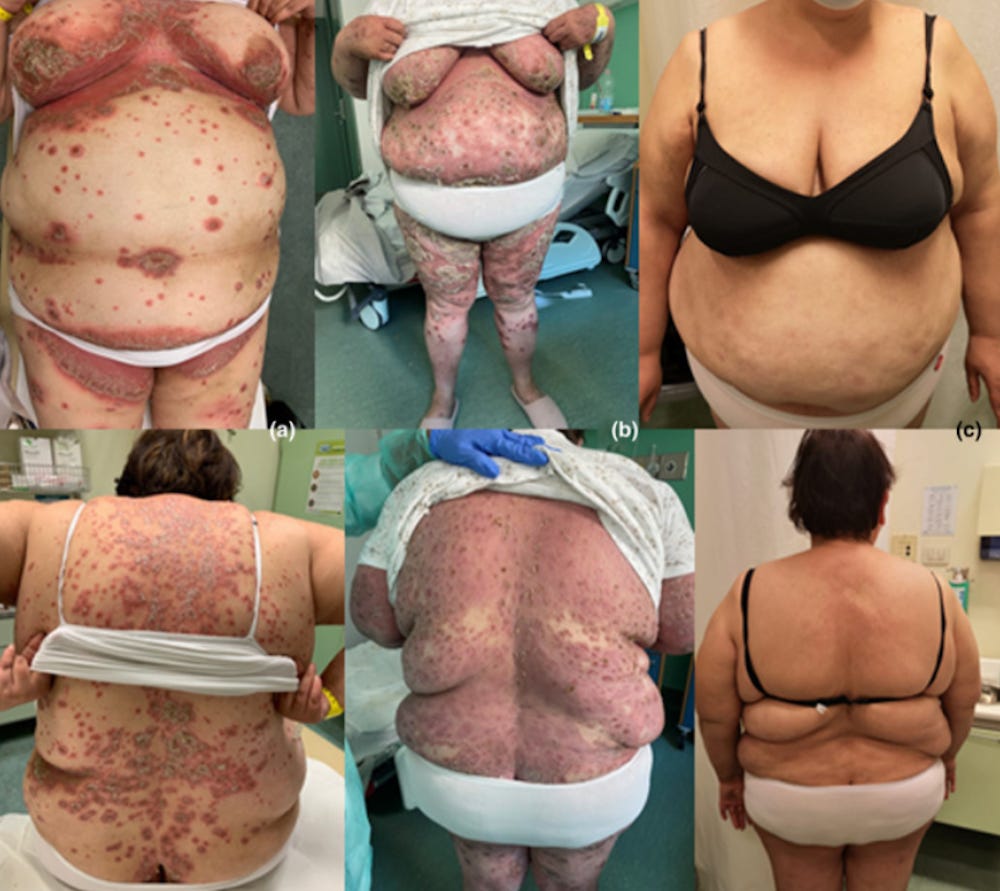
35237629 Case Report: Rowell Syndrome-Like Flare of Cutaneous Lupus Erythematosus Following COVID-19 Infection
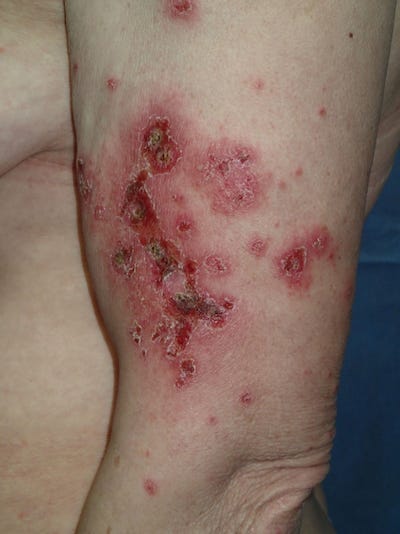
35251600 Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) Covid-19 vaccine: A case report
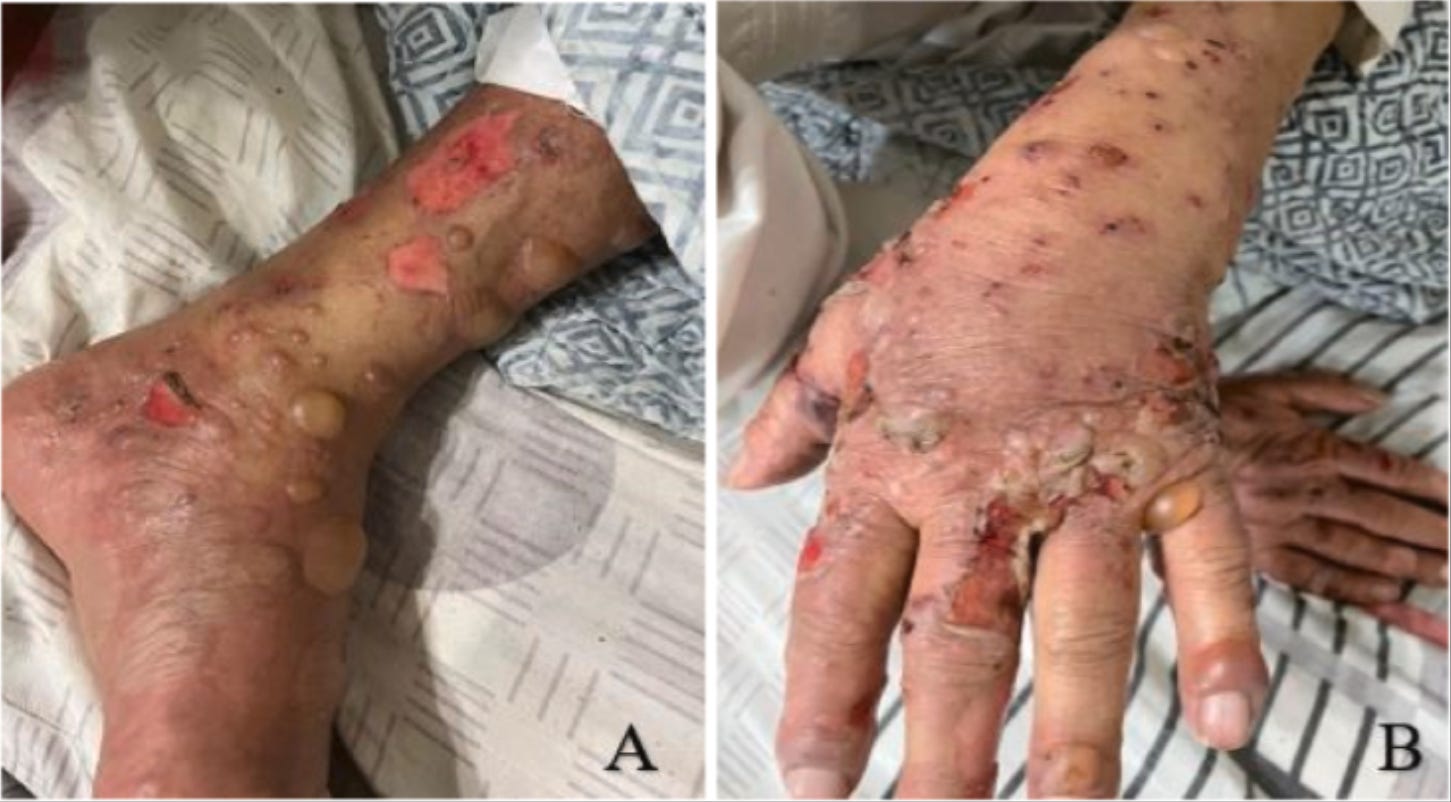
35308065 A case report of anti-P200 pemphigoid following COVID-19 vaccination
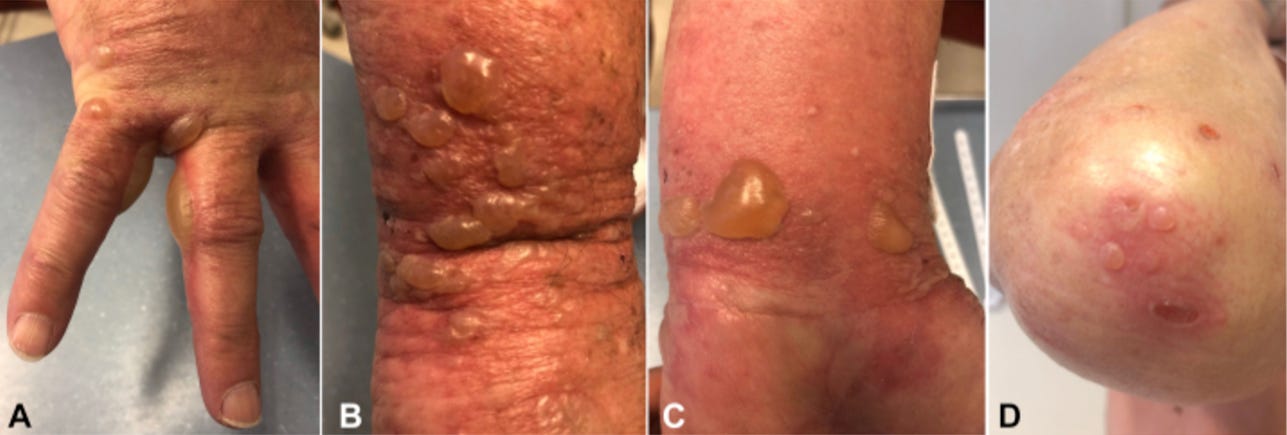
35299937 A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis
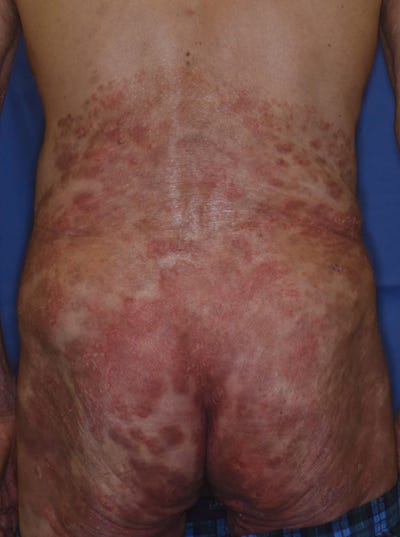
35331228 Oral erythema multiforme after Pfizer-BioNTech COVID-19 vaccination: a report of four cases
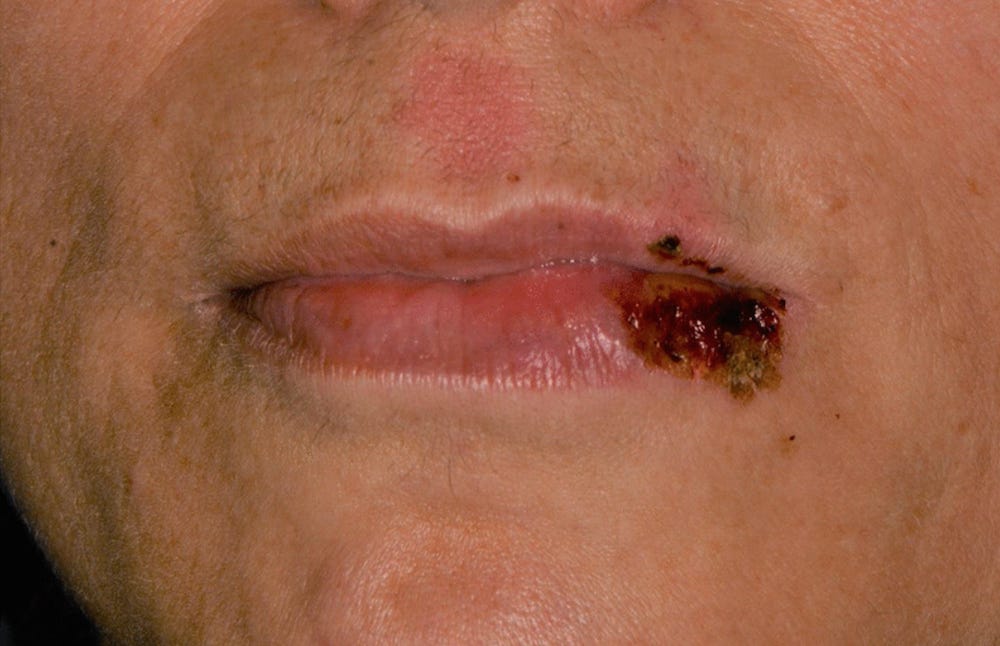
35425676 Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Series of Five Cases
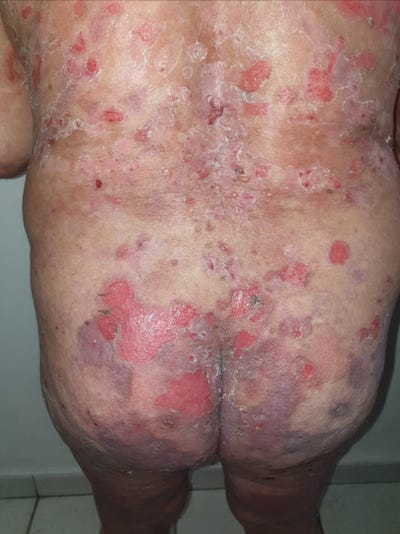
35571457 Toxic epidermal necrolysis-like linear IgA bullous dermatosis after third Moderna COVID-19 vaccine in the setting of oral terbinafine
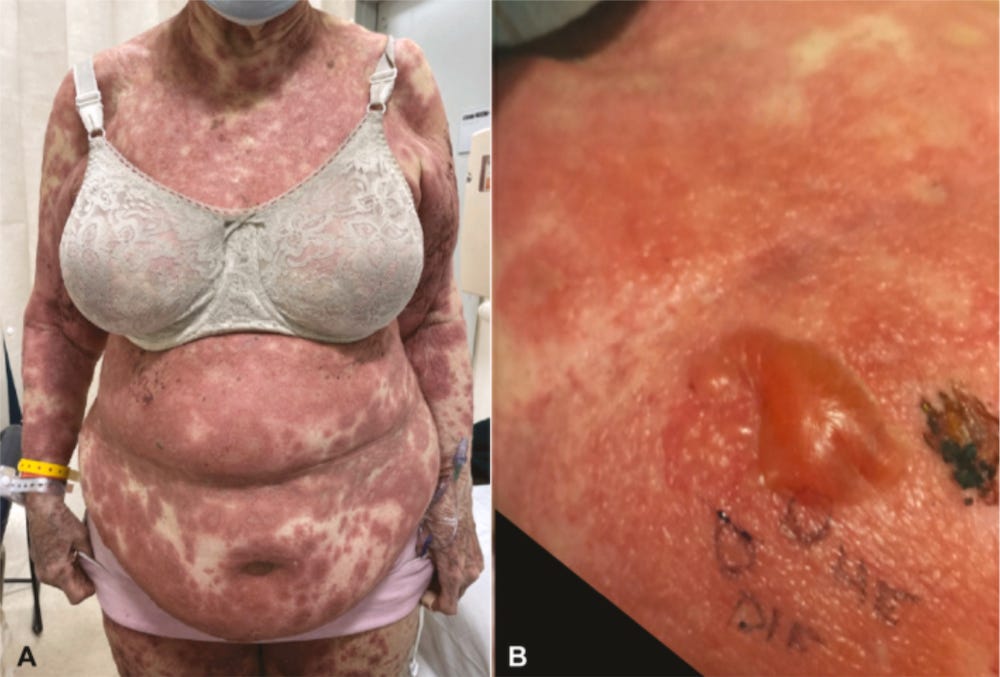

There are many, many, many more horror stories.

https://childrenshealthdefense.org/defender/covid-index-new-research-tool-find-censored-science/

https://covidindex.science/topics
https://covidindex.science/topics/adverse-events
https://covidindex.science/topics/breakthrough-cases
https://CovidIndex.science/topics/covid-19
https://CovidIndex.science/topics/lipid-nanoparticles
https://CovidIndex.science/topics/mrna
https://CovidIndex.science/topics/sars-cov-2-spike-protein

PHMPT.org (Clinical Trial Data)
This nonprofit, made up of public health professionals, medical professionals, scientists, and journalists exists solely to obtain and disseminate the data relied upon by the FDA to license COVID-19 vaccines. The organization takes no position on the data other than that it should be made publicly available to allow independent experts to conduct their own review and analyses.
The US Food and Drug Administration (FDA) attempted to delay the release of Pfizer’s COVID-19 vaccine safety data for 75 years despite approving the injection after only 108 days of safety review on December 11th, 2020.
But in early January 2022, Federal Judge Mark Pittman ordered them to release 55,000 pages per month. They released 12,000 pages by the end of January.
PHMPT has posted all of the documents on its website.
https://phmpt.org/pfizer-16-plus-documents/
https://phmpt.org/pfizer-12-15-documents/
https://phmpt.org/moderna-documents/
https://www.globalresearch.ca/wp-content/uploads/2023/05/pfizer-report.pdf


From Dr. Mark Trozzi:

Many thanks to Dr. Mark Trozzi for compiling the information below.
CLICK HERE to read his entire article.
- Acute Hyperactive Encephalopathy
- Acute Kidney Injury
- Acute Myelitis
- Allergic Reactions
- Alopecia Areata
- Anaphylaxis
- Axillary Adenopathy
- Bell’s Palsy
- Bullous Drug Eruption
- Capillary Leak Syndrome
- Cardiac Complications
- Central Serous Retinopathy
- Cerebral Venous Thrombosis
- Cutaneous Adverse Effects
- Facial Nerve Palsy
- Guillain-Barré Syndrome
- Hemophagocytic Lymphohistiocytosis
- Henoch-Schonlein Purpura
- Immune-Mediated Disease Outbreaks
- Immune-Mediated Hepatitis
- Internal Bleeding
- Intracerebral Haemorrhage
- Lymphadenopathy
- Multiple Sclerosis
- Myocarditis
- Myopericarditis
- Nephrotic Syndrome
- Neurological Symptoms
- Oculomotor Paralysis
- Pericarditis
- Perimyocarditis
- Petechiae
- Prion Disease
- Psoriasis
- Pulmonary Embolism
- Purpura Annularis Telangiectodes
- Rhabdomyolysis
- Systemic Lupus Erythematosus
- Takotsubo Cardiomyopathy
- Thrombocytopenia
- Thrombosis
- Thrombotic Thrombocytopenic Purpura
- Vasculitis
- Vogt-Koyanagi-Harada Syndrome
REFERENCES
1 Acute Hyperactive Encephalopathy references
- Acute Hyperactive Encephalopathy Following COVID-19 Vaccination with Dramatic Response to Methylprednisolone: A Case Report
- Post-vaccinal Encephalitis after ChAdOx1 nCov-19
- Acute Disseminated Encephalomyelitis Following Vaccination Against SARS-CoV-2
- Acute Hyperactive Encephalopathy Following COVID-19 Vaccination with Dramatic Response to Methylprednisolone: Case Report
3 Acute Myelitis references
- Acute Myelitis and ChAdOx1 nCoV-19 Vaccine: Coincidental or Causal Association
- Acute Transverse Myelitis (ATM): Clinical Review of 43 Patients with COVID-19-Associated ATM and 3 Serious Adverse Events of Post-Vaccination ATM with ChAdOx1 nCoV-19 Vaccine (AZD1222)
- Transverse Myelitis Induced by SARS-CoV-2 Vaccination
- Acute Transverse Myelitis (ATM): Clinical Review of 43 Patients with COVID-19-Associated ATM and 3 Serious Adverse Events of Post-Vaccination ATM with ChAdOx1 nCoV-19 (AZD1222) Vaccine
- Acute Transverse Myelitis After COVID-19 Vaccination
- Extensive Longitudinal Transverse Myelitis After ChAdOx1 nCOV-19 Vaccine: Case Report
- Acute Transverse Myelitis After SARS-CoV-2 Vaccination: Case Report and Review of the Literature
- Acute Transverse Myelitis Following Inactivated COVID-19 Vaccine
- Acute Transverse Myelitis After COVID-19 Vaccination
- A Case of Longitudinally Extensive Transverse Myelitis Following Covid-19 Vaccination
- Post COVID-19 Transverse Myelitis; A Case Report with Review of the Literature
- Acute Bilateral Optic Neuritis/Chiasm with Longitudinal Extensive Transverse Myelitis in Long-standing Stable Multiple Sclerosis After Vector-Based Vaccination Against SARS-CoV-19
- Extensive Longitudinal Transverse Myelitis Following AstraZeneca COVID-19 Vaccination
- Extensive Longitudinal Transverse Myelitis Following AstraZeneca COVID-19 Vaccination
- Longitudinally Extensive Cervical Myelitis After Vaccination with Inactivated Virus Based COVID-19 Vaccine
4 Allergic Reactions references
- An Academic Hospital Experience Assessing the Risk of COVID-19 mRNA Vaccine Using Patient’s Allergy History
- Allergic Reactions, Including Anaphylaxis, After Receiving the First Dose of the Pfizer-BioNTech COVID-19 Vaccine
- Allergic Reactions to the First COVID-19 Vaccine: A Potential Role of Polyethylene Glycol
- Pfizer Vaccine Raises Allergy Concerns
- Allergic Reactions, Including Anaphylaxis, After Receiving the First Dose of Pfizer-BioNTech COVID-19 Vaccine – United States, December 14-23, 2020
- Allergic Reactions, Including Anaphylaxis, After Receiving First Dose of Modern COVID-19 Vaccine – United States, December 21, 2020-January 10, 2021
- Severe Allergic Reactions After COVID-19 Vaccination with the Pfizer/BioNTech Vaccine in Great Britain and the USA: Position Statement of the German Allergy Societies
- Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines
- Allergenic Components of the mRNA-1273 Vaccine for COVID-19: Possible Involvement of Polyethylene Glycol and IgG-Mediated Complement Activation
- Acute Allergic Reactions to COVID-19 mRNA Vaccines
- Polyethylene Glycol Allergy of the SARS CoV2 Vaccine Recipient: Case Report of a Young Adult Recipient and Management of Future Exposure to SARS-CoV2
- Allergic Reactions and Adverse Events Associated with Administration of mRNA-Based Vaccines. A Health System Experience
- Allergic Reactions to COVID-19 Vaccines: Statement of the Belgian Society of Allergy and Clinical Immunology (BelSACI)
- Allergic Reactions After COVID-19 Vaccination: Putting the Risk in Perspective
- Risk of Severe Allergic Reactions to COVID-19 Vaccines Among Patients with Allergic Skin Disease: Practical Recommendations. An ETFAD Position Statement with External Experts
- Association of Self-Reported History of High-Risk Allergy with Allergy Symptoms After COVID-19 Vaccination
- The Risk of Allergic Reaction to SARS-CoV-2 Vaccines and Recommended Evaluation and Management: A Systematic Review, Meta-Analysis, GRADE Assessment, and International Consensus Approach
- Practical Handling of Allergic Reactions to COVID-19 Vaccines: A Position Paper from German and Austrian Allergy Societies AeDA, DGAKI, GPA and OGAI
- Severe Allergic Reactions After COVID-19 Vaccination with the Pfizer/BioNTech Vaccine in Great Britain and USA: Position Statement of the German Allergy Societies
- Assessment of Allergic and Anaphylactic Reactions to mRNA COVID-19 Vaccines with Confirmatory Testing in a US Regional Health System
- A Case Series of Skin Reactions to COVID-19 Vaccine in the Department of Dermatology at Loma Linda University
- Skin Reactions Reported After Moderna and Pfizer’s COVID-19 Vaccination: A Study Based on a Registry of 414 Cases
- Skin Reactions After Vaccination Against SARS-CoV-2: A Nationwide Spanish Cross-Sectional Study of 405 Cases
- Coagulopathies After SARS-CoV-2 Vaccination May Derive from a Combined Effect of SARS-CoV-2 Spike Protein and Adenovirus Vector-Activated Signaling Pathways
- Diffuse Prothrombotic Syndrome After Administration of ChAdOx1 nCoV-19 Vaccine: Case Report
- Concerning the Unexpected Prothrombotic State Following Some Coronavirus Disease 2019 Vaccines (Calcaterra, G., et al.)
- Post-Vaccination Multisystem Inflammatory Syndrome in Adults Without Evidence of Prior SARS-CoV-2 Infection
- Autoantibody Release in Children After Coronavirus mRNA Vaccination: A Risk Factor of Multisystem Inflammatory Syndrome? (Buchhorn, R., et al.)
6 Anaphylaxis references
- COVID-19 Vaccine-Associated Anaphylaxis: A Statement from the Anaphylaxis Committee of the World Allergy Organization
- Allergic Reactions, Including Anaphylaxis, After Receiving the First Dose of the Pfizer-BioNTech COVID-19 Vaccine
- Allergic Reactions, Including Anaphylaxis, After Receiving the First Dose of Pfizer-BioNTech COVID-19 Vaccine – United States, December 14-23, 2020
- Allergic Reactions, Including Anaphylaxis, After Receiving First Dose of Modern COVID-19 Vaccine – United States, December 21, 2020-January 10, 2021
- Reports of Anaphylaxis After Coronavirus Disease Vaccination 2019, South Korea, February 26-April 30, 2021
- Reports of Anaphylaxis After Receiving COVID-19 mRNA Vaccines in the U.S.-Dec 14, 2020-Jan 18, 2021
- Immunization Practices and Risk of Anaphylaxis: A Current, Comprehensive Update of COVID-19 Vaccination Data
- Relationship Between Pre-existing Allergies and Anaphylactic Reactions Following Administration of COVID-19 mRNA Vaccine
- Anaphylaxis Associated with COVID-19 mRNA Vaccines: Approach to Allergy Research
- Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines
- Cumulative Adverse Event Report of Anaphylaxis Following Injections of COVID-19 mRNA Vaccine (Pfizer-BioNTech) in Japan: The First Month Report
- COVID-19 Vaccines Increase the Risk of Anaphylaxis
- Biphasic Anaphylaxis After Exposure to the First Dose of the Pfizer-BioNTech COVID-19 mRNA Vaccine COVID-19
- Polyethylene Glycol (PEG) Is a Cause of Anaphylaxis to Pfizer/BioNTech mRNA COVID-19 Vaccine
- Elevated Rates of Anaphylaxis After Vaccination With Pfizer BNT162b2 mRNA Vaccine Against COVID-19 in Japanese Healthcare Workers; A Secondary Analysis of Initial Post-Approval Safety Data
- IgE-Mediated Allergy to Polyethylene Glycol (PEG) as a Cause of Anaphylaxis to COVID-19 mRNA Vaccines
- Anaphylactic Reactions to COVID-19 mRNA Vaccines: A Call for Further Studies
- Anaphylaxis Following COVID-19 Vaccine in a Patient With Cholinergic Urticaria
- Anaphylaxis Induced by CoronaVac COVID-19 Vaccine: Clinical Features and Results of Revaccination
- Anaphylaxis After Modern COVID-19 Vaccine
- Sex Differences in the Incidence of Anaphylaxis to LNP-mRNA Vaccines COVID-19
- Allergic Reactions, Including Anaphylaxis, After Receiving the First Dose of Pfizer-BioNTech COVID-19 Vaccine – United States, December 14 to 23, 2020
- Allergic Reactions, Including Anaphylaxis, After Receiving the First Dose of Modern COVID-19 Vaccine – United States, December 21, 2020 to January 10, 2021
- Prolonged Anaphylaxis to Pfizer 2019 Coronavirus Disease Vaccine: A Case Report and Mechanism of Action
- Anaphylaxis Reactions to Pfizer BNT162b2 Vaccine: Report of 3 Cases of Anaphylaxis Following Vaccination with Pfizer BNT162b2
- Biphasic Anaphylaxis After First Dose of 2019 Messenger RNA Coronavirus Disease Vaccine with Positive Polysorbate 80 Skin Test Result
- Biphasic Anaphylaxis After Exposure to the First Dose of Pfizer-BioNTech COVID-19 mRNA Vaccine COVID-19
- Cumulative Adverse Event Reporting of Anaphylaxis After mRNA COVID-19 Vaccine (Pfizer-BioNTech) Injections in Japan: The First-Month Report
- Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine – United States, December 14-23, 2020
7 Axillary Adenopathy references
- COVID-19 Vaccine-Induced Axillary and Pectoral Lymphadenopathy in PET
- Evolution of Bilateral Hypermetabolic Axillary Lymphadenopathy on FDG PET/CT After 2-Dose COVID-19 Vaccination
- COVID-19 Vaccine-Related Axillary Lymphadenopathy in Breast Cancer Patients: Case Series With Literature Review
- Subclinical Axillary Lymphadenopathy Associated With COVID-19 Vaccination on Screening Mammography
- Axillary Adenopathy Associated With COVID-19 Vaccination: Imaging Findings and Follow-Up Recommendations in 23 Women
- Unilateral Axillary Adenopathy in the Setting of COVID-19 Vaccination: Follow-Up
- COVID-19 Vaccine-Related Axillary and Cervical Lymphadenopathy in Patients With Current or Previous Breast Cancer and Other Malignancies: Cross-Sectional Imaging Findings on MRI, CT, and PET-CT
- Incidence of Axillary Adenopathy on Breast Imaging After Vaccination with COVID-19
- Unilateral Axillary Lymphadenopathy Related to COVID-19 Vaccine: Pattern on Screening Breast MRI Allowing Benign Evaluation
- Axillary Lymphadenopathy in Patients With Recent COVID-19 Vaccination: A New Diagnostic Dilemma
- COVID-19 Vaccine-Induced Unilateral Axillary Adenopathy: Follow-Up Evaluation in the USA
- Adenopathy After COVID-19 Vaccination
8 Bell’s Palsy references
- Bell’s Palsy and SARS-CoV-2 Vaccines: An Unfolding Story
- Bell’s Palsy After the Second Dose of the Pfizer COVID-19 Vaccine in a Patient With a History of Recurrent Bell’s Palsy
- Bell’s Palsy After COVID-19 Vaccination: Case Report
- The Association Between COVID-19 Vaccination and Bell’s Palsy
- Bell’s Palsy After COVID-19 Vaccination
- Bell’s Palsy After 24 Hours of mRNA-1273 SARS-CoV-2 mRNA-1273 Vaccine
- Bell’s Palsy After Ad26.COV2.S COVID-19 Vaccination
- Bell’s Palsy After COVID-19 Vaccination: Case Report
- Acute Facial Paralysis as a Possible Complication of SARS-CoV-2 Vaccination
- Bell’s Palsy After COVID-19 Vaccination With High Antibody Response in CSF
- Bell’s Palsy After a Single Dose of Vaccine mRNA SARS-CoV-2: Case Report
- Adverse Event Reporting and Risk of Bell’s Palsy After COVID-19 Vaccination
- Bilateral Facial Nerve Palsy and COVID-19 Vaccination: Causality or Coincidence
- Left Bell’s Palsy After the First Dose of mRNA-1273 SARS-CoV-2 Vaccine: Case Report
- Bell’s Palsy After Inactivated Vaccination With COVID-19 in a Patient With a History of Recurrent Bell’s Palsy: Case Report
- Bell’s Palsy After Vaccination With mRNA (BNT162b2) and Inactivated (CoronaVac) SARS-CoV-2 Vaccines: A Case Series and a Nested Case-Control Study
- A Case of Acute Demyelinating Polyradiculoneuropathy With Bilateral Facial Palsy After ChAdOx1 nCoV-19 Vaccine
- Type I Interferons as a Potential Mechanism Linking COVID-19 mRNA Vaccines With Bell’s Palsy
11 Cardiac Complications references
- Transient Cardiac Injury in Adolescents Receiving the BNT162b2 mRNA COVID-19 Vaccine
- Cardiac Complications Following mRNA COVID-19 Vaccines: A Systematic Review of Case Reports and Case Series
- A Review of COVID-19 Vaccination and the Reported Cardiac Manifestations
- Temporal Relationship Between the Second Dose of BNT162b2 mRNA Covid-19 Vaccine and Cardiac Involvement in a Patient With Previous SARS-COV-2 Infection
- Autopsy Findings and Causality Relationship Between Death and COVID-19 Vaccination: A Systematic Review
- Post-Mortem Investigation of Deaths After Vaccination with COVID-19 Vaccines
- A Look at the Role of Postmortem Immunohistochemistry in Understanding the Inflammatory Pathophysiology of COVID-19 Disease and Vaccine-Related Thrombotic Adverse Events: A Narrative Review
- COVID-19 Vaccine and Death: Causality Algorithm According to the WHO Eligibility Diagnosis
- Myocarditis and Other Cardiovascular Complications of COVID-19 mRNA-based COVID-19 Vaccines
- Cardiovascular Magnetic Resonance Imaging Findings in Young Adult Patients with Acute Myocarditis after COVID-19 mRNA Vaccination: A Case Series
- Be Alert to the Risk of Adverse Cardiovascular Events after COVID-19 Vaccination
- Myocarditis and Other Cardiovascular Complications of mRNA-based COVID-19 Vaccines
13 Cerebral Venous Thrombosis References
- Cerebral Venous Sinus Thrombosis in the U.S. Population After SARS-CoV-2 Vaccination with Adenovirus and After COVID-19
- Cerebral Venous Sinus Thrombosis Negative for Anti-PF4 Antibody Without Thrombocytopenia After Immunization with COVID-19 Vaccine in a Non-Comorbid Elderly Indian Male Treated with Conventional Heparin-Warfarin Based Anticoagulation
- Cerebral Venous Thrombosis After BNT162b2 mRNA SARS-CoV-2 Vaccine
- Cerebral Venous Sinus Thrombosis After Vaccination: The United Kingdom Experience
- US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Vaccination with Ad26.COV2.S (against COVID-19), March 2 to April 21, 2020
- Management of Cerebral and Splanchnic Vein Thrombosis Associated with Thrombocytopenia in Subjects Previously Vaccinated with Vaxzevria (AstraZeneca): Position Statement of the Italian Society for the Study of Hemostasis and Thrombosis (SISET)
- Vaccine-Induced Immune Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After Vaccination with COVID-19: A Systematic Review
- Early Results of Bivalirudin Treatment for Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After Vaccination with COV2.S
- A Rare Case of a Middle-Aged Asian Male with Cerebral Venous Thrombosis After AstraZeneca COVID-19 Vaccination
- Cerebral Venous Sinus Thrombosis and Thrombocytopenia After COVID-19 Vaccination: Report of Two Cases in the United Kingdom
- Diagnostic-Therapeutic Recommendations of the Ad-Hoc FACME Expert Working Group on the Management of Cerebral Venous Thrombosis Related to COVID-19 Vaccination
- COVID-19 Vaccination: Information on the Occurrence of Arterial and Venous Thrombosis Using Data from VigiBase
- Cerebral Venous Thrombosis Associated with the COVID-19 Vaccine in Germany
- Cerebral Venous Thrombosis Following BNT162b2 mRNA Vaccination of BNT162b2 Against SARS-CoV-2: A Black Swan Event
- The Importance of Recognizing Cerebral Venous Thrombosis Following Anti-COVID-19 Vaccination
- Cerebral Venous Sinus Thrombosis Negative for Anti-PF4 Antibody Without Thrombocytopenia After Immunization with COVID-19 Vaccine in an Elderly, Non-Comorbid Indian Male Treated with Conventional Heparin-Warfarin-Based Anticoagulation
- Vaccine-Induced Immune Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination: A Systematic Review
- A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated with Administration of COVID-19 Vaccine
- Acute Cerebral Venous Thrombosis and Pulmonary Artery Embolism Associated with the COVID-19 Vaccine
- Cerebral Venous Sinus Thrombosis and Vaccine-Induced Thrombocytopenia: A Missed Opportunity for a Rapid Return on Experience
- Diagnosis and Treatment of Cerebral Venous Sinus Thrombosis with Vaccine-Induced Immune Thrombotic Thrombocytopenia
- Venous Sinus Thrombosis After Vaccination with ChAdOx1 nCov-19
- Cerebral Venous Thrombosis Following Vaccination Against SARS-CoV-2: An Analysis of Cases Reported to the European Medicines Agency
- Arterial Events, Venous Thromboembolism, Thrombocytopenia, and Bleeding After Vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population-Based Cohort Study
- Cerebral Venous Thrombosis Associated with COVID-19 Vaccine in Germany
- Malignant Cerebral Infarction After Vaccination with ChAdOx1 nCov-19: A Catastrophic Variant of Vaccine-Induced Immune-Mediated Thrombotic Thrombocytopenia
- Cerebral Venous Sinus Thrombosis Associated with Thrombocytopenia After COVID-19 Vaccination
- Central Venous Sinus Thrombosis with Subarachnoid Hemorrhage After COVID-19 mRNA Vaccination: Are These Reports Merely Coincidental?
- Cerebral Venous Sinus Thrombosis Negative for Anti-PF4 Antibody Without Thrombocytopenia After Immunization with COVID-19 Vaccine in a Non-Comorbid Elderly Indian Male Treated with Conventional Heparin-Warfarin-Based Anticoagulation
- Cerebral Venous Sinus Thrombosis 2 Weeks After First Dose of SARS-CoV-2 mRNA Vaccine
- Deep Venous Thrombosis (DVT) Occurring Shortly After the Second Dose of SARS-CoV-2 mRNA Vaccine
- Vaccine-Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis with High Mortality Rate: A Case Series
- Procoagulant Microparticles: A Possible Link Between Vaccine-Induced Immune Thrombocytopenia (VITT) and Cerebral Sinus Venous Thrombosis
- Acute Cerebral Venous Thrombosis and Pulmonary Artery Embolism Associated with the COVID-19 Vaccine
- Cerebral Venous Thrombosis Following COVID-19 Vaccination
- Adverse Effects Reported After COVID-19 Vaccination in a Tertiary Care Hospital, Centered on Cerebral Venous Sinus Thrombosis (CVST)
- Cerebral Venous Thrombosis Associated with COVID-19 Vaccine in Germany
- Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination: Neurological and Radiological Management
- Cerebral Venous Thrombosis and Thrombocytopenia After COVID-19 Vaccination
- Cerebral Venous Sinus Thrombosis and Thrombocytopenia After COVID-19 Vaccination: Report of Two Cases in the United Kingdom
- Cerebral Venous Thrombosis Induced by SARS-CoV-2 Vaccine
- Cerebral Venous Sinus Thrombosis Associated with Vaccine-Induced Thrombotic Thrombocytopenia
- Cerebral Venous Thrombosis After the BNT162b2 mRNA SARS-CoV-2 Vaccine
- Cerebral Venous Thrombosis After COVID-19 Vaccination
- Lethal Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination
- Cerebral Venous Sinus Thrombosis in the U.S. Population After SARS-CoV-2 Vaccination with Adenovirus and After COVID-19
- Cerebral Venous Thrombosis After COVID-19 Vaccination: Is the Risk of Thrombosis Increased by Intravascular Administration of the Vaccine
- Central Venous Sinus Thrombosis with Subarachnoid Hemorrhage After COVID-19 mRNA Vaccination: Are These Reports Merely Coincidental?
- Cerebral Venous Sinus Thrombosis After ChAdOx1 nCov-19 Vaccination with a Misleading First Brain MRI
- Early Results of Bivalirudin Treatment for Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After Vaccination with Ad26.COV2.S
- Cerebral Venous Sinus Thrombosis Associated with Post-Vaccination Thrombocytopenia by COVID-19
- Cerebral Venous Sinus Thrombosis 2 Weeks After the First Dose of SARS-CoV-2 mRNA Vaccine
- Adverse Effects Reported After COVID-19 Vaccination in a Tertiary Care Hospital, Focus on Cerebral Venous Sinus Thrombosis (CVST)
- Cerebral Venous Sinus Thrombosis Following Vaccination Against SARS-CoV-2: An Analysis of Cases Reported to the European Medicines Agency
- A Rare Case of a Middle-Age Asian Male with Cerebral Venous Thrombosis After COVID-19 AstraZeneca Vaccination
- Massive Cerebral Venous Thrombosis and Venous Basin Infarction as Late Complications of COVID-19: A Case Report
- Massive Cerebral Venous Thrombosis Due to Vaccine-Induced Immune Thrombotic Thrombocytopenia
- Cerebral Venous Thrombosis Developing After Vaccination, COVID-19: VITT, VATT, TTS and More
- Cerebral Venous Thrombosis and Myeloproliferative Neoplasms: A Three-Center Study of 74 Consecutive Cases
- Vaccine-Induced Cerebral Venous Thrombosis and Thrombocytopenia, Oxford-AstraZeneca COVID-19: A Missed Opportunity for Rapid Return on Experience
- Case Report: Take a Second Look – Cerebral Venous Thrombosis Related to Covid-19 Vaccination and Thrombotic Thrombocytopenia Syndrome
14 Cutaneous Adverse Effects references
- Cutaneous Adverse Effects of Available COVID-19 Vaccines
- Rare Cutaneous Adverse Effects of COVID-19 Vaccines: A Case Series and Review of the Literature
- Cutaneous Adverse Reactions of 35,229 Doses of COVID-19 Sinovac and AstraZeneca Vaccine COVID-19: A Prospective Cohort Study in Health Care Workers
15 Facial Nerve Palsy references
- Facial Nerve Palsy Following Administration of COVID-19 mRNA Vaccines: Analysis of Self-Report Database
- COVID-19 Vaccination Association and Facial Nerve Palsy: A Case-Control Study
- Sequential Contralateral Facial Nerve Palsy After First and Second Doses of COVID-19 Vaccine
- Peripheral Facial Nerve Palsy After Vaccination with BNT162b2 (COVID-19)
- Facial Nerve Palsy After Administration of COVID-19 mRNA Vaccines: Analysis of Self-Report Database
- A Case of Acute Demyelinating Polyradiculoneuropathy with Bilateral Facial Palsy Following ChAdOx1 nCoV-19 Vaccination
16 Guillain-Barré Syndrome references
- GM1 Ganglioside Antibody and COVID-19-Related Guillain-Barre Syndrome: Case Report, Systemic Review, and Implications for Vaccine Development
- Guillain-Barré Syndrome After AstraZeneca COVID-19 Vaccination: Causal or Casual Association
- Sensory Guillain-Barré Syndrome After ChAdOx1 nCov-19 Vaccine: Report of Two Cases and Review of the Literature
- Guillain-Barré Syndrome After the First Dose of SARS-CoV-2 Vaccine: A Temporary Occurrence, Not a Causal Association
- Guillain-Barré Syndrome Presenting as Facial Diplegia After Vaccination With COVID-19: A Case Report
- Guillain-Barré Syndrome After the First Injection of ChAdOx1 nCoV-19 Vaccine: First Report
- SARS-CoV-2 Vaccines Are Not Safe for Those With Guillain-Barre Syndrome Following Vaccination
- Guillain Barré Syndrome After Vaccination with mRNA-1273 Against COVID-19
- A Novel Case of Bifacial Diplegia Variant of Guillain-Barré Syndrome After Vaccination With Janssen COVID-19
- Sensory Guillain-Barré Syndrome Following ChAdOx1 nCov-19 Vaccine: Report of Two Cases and Review of the Literature
- Facial Diplegia: A Rare and Atypical Variant of Guillain-Barré Syndrome and the Ad26.COV2.S Vaccine
- Guillain-Barré Syndrome After ChAdOx1 nCoV-19 COVID-19 Vaccination: A Case Series
- AstraZeneca COVID-19 Vaccine and Guillain-Barré Syndrome in Tasmania: A Causal Link
- COVID-19, Guillain-Barré and Vaccine: A Dangerous Mix
- Guillain-Barré Syndrome After the First Dose of Pfizer-BioNTech COVID-19 Vaccine: Case Report and Review of Reported Cases
- Guillain-Barre Syndrome After BNT162b2 COVID-19 Vaccine
- COVID-19 Adenovirus Vaccines and Guillain-Barré Syndrome with Facial Palsy
- Association of Receipt Association of Ad26.COV2.S COVID-19 Vaccine with Presumed Guillain-Barre Syndrome, February-July 2021
- A Case of Guillain-Barré Syndrome After Pfizer-BioNTech COVID-19 Vaccine
- Guillain-Barré Syndrome Associated with COVID-19 Vaccination
- Rate of Recurrent Guillain-Barré Syndrome After COVID-19 BNT162b2 mRNA Vaccine
- Guillain-Barre Syndrome After COVID-19 Vaccination in an Adolescent
- Guillain-Barre Syndrome After ChAdOx1-S / nCoV-19 Vaccination
- Guillain-Barre Syndrome After COVID-19 mRNA-1273 Vaccine: Case Report
- Guillain-Barre Syndrome Following SARS-CoV-2 Vaccination in 19 Patients
- Guillain-Barre Syndrome Presenting With Facial Diplegia Following Vaccination With COVID-19 in Two Patients
- A Rare Case of Guillain-Barré Syndrome After COVID-19 Vaccination
- Neurological Complications of COVID-19: Guillain-Barre Syndrome After Pfizer COVID-19 Vaccine
- COVID-19 Vaccine Causing Guillain-Barre Syndrome, an Uncommon Potential Side Effect
- Guillain-Barre Syndrome After the First Dose of COVID-19 Vaccination: Case Report
- Guillain-Barre Syndrome After the First Injection of ChAdOx1 nCoV-19 Vaccine: First Report
- A Case of Sensory Ataxic Guillain-Barre Syndrome With Immunoglobulin G Anti-GM1 Antibodies After First Dose of COVID-19 BNT162b2 mRNA Vaccine (Pfizer)
- A Variant of Guillain-Barré Syndrome After SARS-CoV-2 Vaccination: AMSAN
- A Rare Variant of Guillain-Barré Syndrome After Vaccination With Ad26.COV2.S
- Guillain-Barré Syndrome After SARS-CoV-2 Vaccination in a Patient With Previous Vaccine-Associated Guillain-Barré Syndrome
- Guillain-Barré Syndrome in an Australian State Using mRNA and Adenovirus-Vector SARS-CoV-2 Vaccines
- Variant Guillain-Barré Syndrome Occurring After SARS-CoV-2 Vaccination
- Guillain-Barre Syndrome With Axonal Variant Temporally Associated With Modern SARS-CoV-2 mRNA-Based Vaccine
- Guillain-Barre Syndrome After the First Dose of SARS-CoV-2 Vaccine: A Temporary Occurrence, Not a Causal Association
- SARS-CoV-2 Vaccines Can Be Complicated Not Only by Guillain-Barré Syndrome But Also by Distal Small Fiber Neuropathy
- Clinical Variant of Guillain-Barré Syndrome With Prominent Facial Diplegia After AstraZeneca 2019 Coronavirus Disease Vaccine
- Miller-Fisher Syndrome and Guillain-Barré Syndrome Overlap Syndrome in a Patient After Oxford-AstraZeneca SARS-CoV-2 Vaccination
- Bilateral Facial Weakness With a Variant of Paresthesia of Guillain-Barre Syndrome After Vaxzevria COVID-19 Vaccine
18 Henoch-Schonlein Purpura references
- A Rare Case of Henoch-Schönlein Purpura after a Case Report of COVID-19 Vaccine
- Henoch-Schönlein Purpura Occurring after Vaccination with COVID-19
- Henoch-Schönlein Purpura following the First Dose of COVID-19 Viral Vector Vaccine: Case Report
19 Immune-Mediated Disease Outbreaks references
- Lobar Hemorrhage with Ventricular Rupture Shortly After the First Dose of an mRNA-Based SARS-CoV-2 Vaccine
- Retinal Hemorrhage After SARS-CoV-2 Vaccination
- Lobar Hemorrhage with Ventricular Rupture Shortly After the First Dose of a SARS-CoV-2 mRNA-Based SARS-CoV-2 Vaccine
- Acral Hemorrhage After Administration of the Second Dose of SARS-CoV-2 Vaccine. A Post-Vaccination Reaction
- Fatal Cerebral Hemorrhage After COVID-19 Vaccine
- Intracerebral Hemorrhage Associated with Vaccine-Induced Thrombotic Thrombocytopenia After ChAdOx1 nCOVID-19 Vaccination in a Pregnant Woman
20 Immune-Mediated Hepatitis references
- Autoimmune Hepatitis Developing After Coronavirus Disease Vaccine 2019 (COVID-19): Causality or Victim?
- Autoimmune Hepatitis Triggered by Vaccination Against SARS-CoV-2
- Acute Autoimmune-Like Hepatitis With Atypical Antimitochondrial Antibody After Vaccination With COVID-19 mRNA: A New Clinical Entity
- Autoimmune Hepatitis After COVID Vaccine
- Hepatitis C Virus Reactivation After COVID-19 Vaccination: A Case Report
- Autoimmune Hepatitis Developing After ChAdOx1 nCoV-19 Vaccine (Oxford-AstraZeneca)
- Autoimmune Hepatitis Triggered by SARS-CoV-2 Vaccination
- Immune-Mediated Hepatitis With the Moderna Vaccine Is No Longer a Coincidence but Confirmed
22 Intracerebral Haemorrhage references
- Intracerebral Haemorrhage Due to Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination: The First Fatal Case in Korea
- Intracerebral Haemorrhage Twelve Days After Vaccination With ChAdOx1 nCoV-19
- Neurosurgical Considerations Regarding Decompressive Craniectomy for Intracerebral Hemorrhage After SARS-CoV-2 Vaccination in Vaccine-Induced Thrombotic Thrombocytopenia-VITT
- First Dose of ChAdOx1 and BNT162b2 COVID-19 Vaccines and Thrombocytopenic, Thromboembolic, and Hemorrhagic Events in Scotland
- Large Hemorrhagic Stroke After Vaccination Against ChAdOx1 nCoV-19: A Case Report
- Major Hemorrhagic Stroke After ChAdOx1 nCoV-19 Vaccination: A Case Report
- Aphasia Seven Days After the Second Dose of an mRNA-Based SARS-CoV-2 Vaccine. Brain MRI Revealed an Intracerebral Haemorrhage (ICBH) in the Left Temporal Lobe in a 52-Year-Old Man
- Incidence of Acute Ischemic Stroke After Coronavirus Vaccination in Indonesia: Case Series
23 Lymphadenopathy references
- Rare Case of Contralateral Supraclavicular Lymphadenopathy After Vaccination With COVID-19: Computed Tomography and Ultrasound Findings
- COVID-19 mRNA Vaccination-Induced Lymphadenopathy Mimics Lymphoma Progression on FDG PET/CT
- Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncology Patients
- Hypermetabolic Lymphadenopathy After Administration of BNT162b2 mRNA Vaccine COVID-19: Incidence Assessed by [18 F] FDG PET-CT and Relevance for Study Interpretation
- Lymphadenopathy After COVID-19 Vaccination: Review of Imaging Findings
- Lymphadenopathy Associated With COVID-19 Vaccination on FDG PET/CT: Distinguishing Features in Adenovirus-Vectored Vaccine
- COVID-19 Vaccination-Induced Lymphadenopathy in a Specialized Breast Imaging Clinic in Israel: Analysis of 163 Cases
- Coronavirus Disease Vaccine 2019 Mimics Lymph Node Metastases in Patients Undergoing Skin Cancer Follow-Up: A Single-Center Study
- COVID-19 Post-Vaccination Lymphadenopathy: Report of Fine-Needle Aspiration Biopsy Cytologic Findings
- Regional Lymphadenopathy After COVID-19 Vaccination: Review of the Literature and Considerations for Patient Management in Breast Cancer Care
- Adverse Events of COVID Injection That May Occur in Children. Acute-Onset Supraclavicular Lymphadenopathy Coincident With Intramuscular mRNA Vaccination Against COVID-19 May Be Related to the Injection Technique of the Vaccine, Spain, January and February 2021
- Supraclavicular Lymphadenopathy After COVID-19 Vaccination in Korea: Serial Follow-Up by Ultrasonography
- Oxford-AstraZeneca COVID-19 Vaccination Induced Lymphadenopathy on [18F] Choline PET/CT, Not Just an FDG Finding
- A Case of Cervical Lymphadenopathy Following COVID-19 Vaccination
- Unilateral Lymphadenopathy After COVID-19 Vaccination: A Practical Management Plan for Radiologists of All Specialties
- Supraclavicular Lymphadenopathy After COVID-19 Vaccination: An Increasing Presentation in the Two-Week Wait Neck Lump Clinic
- COVID-19 Vaccination and Lower Cervical Lymphadenopathy in Two-Week Neck Lump Clinic: A Follow-Up Audit
- Cervical Lymphadenopathy After Coronavirus Disease Vaccination 2019: Clinical Features and Implications for Head and Neck Cancer Services
- Lymphadenopathy Associated With the COVID-19 Vaccine
- Evolution of Lymphadenopathy on PET/MRI Massive Cervical Lymphadenopathy Following Vaccination With COVID-19
- Acute-Onset Supraclavicular Lymphadenopathy Coincident With Intramuscular mRNA Vaccination Against COVID-19 May Be Related to the Injection Technique of the Vaccine, Spain, January and February 2021
- Supraclavicular Lymphadenopathy After COVID-19 Vaccination in Korea: Serial Follow-Up by Ultrasonography
- Oxford-AstraZeneca COVID-19 Vaccination Induced Lymphadenopathy on [18F] Choline PET/CT, Not Just an FDG Finding
- A Case of Cervical Lymphadenopathy Following COVID-19 Vaccination
- Unilateral Lymphadenopathy After COVID-19 Vaccination: A Practical Management Plan for Radiologists of All Specialties
- Supraclavicular Lymphadenopathy After COVID-19 Vaccination: An Increasing Presentation in the Two-Week Wait Neck Lump Clinic
- COVID-19 Vaccination and Lower Cervical Lymphadenopathy in Two-Week Neck Lump Clinic: A Follow-Up Audit
- Cervical Lymphadenopathy After Coronavirus Disease Vaccination 2019: Clinical Features and Implications for Head and Neck Cancer Services
- Lymphadenopathy Associated With the COVID-19 Vaccine
- Evolution of Lymphadenopathy on PET/MRI After COVID-19 Vaccination
- Massive Cervical Lymphadenopathy Following Vaccination With COVID-19
- COVID-19 Vaccine-Related Axillary and Cervical Lymphadenopathy in Patients With Current or Previous Breast Cancer and Other Malignancies: Cross-Sectional Imaging Findings on MRI, CT, and PET-CT
- Supraclavicular Lymphadenopathy After COVID-19 Vaccination in Korea: Serial Follow-Up by Ultrasonography
- Evolution of Lymphadenopathy at PET/MRI After COVID-19 Vaccination
24 Multiple Sclerosis references
- Severe Relapse of Multiple Sclerosis after COVID-19 Vaccination: A Case Report
- Acute Relapse and Impaired Immunization after COVID-19 Vaccination in a Patient with Multiple Sclerosis Treated with Rituximab
- Humoral Response Induced by Prime-Boost Vaccination with ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccines in a Patient with Multiple Sclerosis Treated with Teriflunomide
- Initial Clinical Manifestation of Multiple Sclerosis after Immunization with the Pfizer-BioNTech COVID-19 Vaccine
25 Myocarditis References
- Myocarditis after mRNA vaccination against SARS-CoV-2, a case series
- Myocarditis after immunization with COVID-19 mRNA vaccines in members of the US military
- Association of myocarditis with the BNT162b2 messenger RNA COVID-19 vaccine in a case series of children
- Acute symptomatic myocarditis in seven adolescents after Pfizer-BioNTech COVID-19 vaccination
- Myocarditis and pericarditis after vaccination with COVID-19 mRNA: practical considerations for care providers
- Myocarditis, pericarditis and cardiomyopathy after COVID-19 vaccination
- Myocarditis with COVID-19 mRNA vaccines
- Myocarditis and pericarditis after COVID-19 vaccination
- Myocarditis temporally associated with COVID-19 vaccination
- COVID-19 Vaccination Associated with Myocarditis in Adolescents
- Acute myocarditis after administration of BNT162b2 vaccine against COVID-19
- Temporal association between COVID-19 vaccine Ad26.COV2.S and acute myocarditis: case report and review of the literature
- COVID-19 vaccine-induced myocarditis: a case report with review of the literature
- Potential association between COVID-19 vaccine and myocarditis: clinical and CMR findings
- Recurrence of acute myocarditis temporally associated with receipt of coronavirus mRNA disease vaccine 2019 (COVID-19) in a male adolescent
- Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 COVID-19 mRNA vaccination in two patients
- Acute myocarditis after administration of BNT162b2 vaccine
- Lymphohistocytic myocarditis after vaccination with COVID-19 Ad26.COV2.S viral vector
- Myocarditis following vaccination with BNT162b2 in a healthy male
- Acute myocarditis after Comirnaty (Pfizer) vaccination in a healthy male with previous SARS-CoV-2 infection
- Acute myocarditis after vaccination with SARS-CoV-2 mRNA-1273 mRNA
- Acute myocarditis after SARS-CoV-2 vaccination in a 24-year-old man
- A series of patients with myocarditis after vaccination against SARS-CoV-2 with mRNA-1279 and BNT162b2
- COVID-19 mRNA vaccination and myocarditis
- COVID-19 vaccine and myocarditis
- Epidemiology and clinical features of myocarditis/pericarditis before the introduction of COVID-19 mRNA vaccine in Korean children: a multicenter study
- COVID-19 vaccines and myocarditis
- Myocarditis and other cardiovascular complications of COVID-19 mRNA-based COVID-19 vaccines
- Myocarditis and other cardiovascular complications of COVID-19 mRNA-based COVID-19 vaccines
- Myocarditis, pericarditis, and cardiomyopathy after COVID-19 vaccination
- Myocarditis with COVID-19 mRNA vaccines
- Association of myocarditis with COVID-19 mRNA vaccine in children [broken link]
- Association of myocarditis with COVID-19 messenger RNA vaccine BNT162b2 in a case series of children
- Myocarditis after immunization with COVID-19 mRNA vaccines in members of the U.S. military
- Myocarditis occurring after immunization with COVID-19 mRNA-based COVID-19 vaccines
- Myocarditis following immunization with Covid-19 mRNA
- Patients with acute myocarditis after vaccination withCOVID-19 mRNA
- Myocarditis associated with vaccination with COVID-19 mRNA
- Symptomatic Acute Myocarditis in 7 Adolescents after Pfizer-BioNTech COVID-19 Vaccination
- Cardiovascular magnetic resonance imaging findings in young adult patients with acute myocarditis after COVID-19 mRNA vaccination: a case series
- Clinical Guidance for Young People with Myocarditis and Pericarditis after Vaccination with COVID-19 mRNA
- Cardiac imaging of acute myocarditis after vaccination with COVID-19 mRNA
- Case report: acute myocarditis after the second dose of mRNA-1273 SARS-CoV-2 mRNA vaccine
- Myocarditis / pericarditis associated with COVID-19 vaccine
- The new COVID-19 mRNA vaccine platform and myocarditis: clues to the possible underlying mechanism
- Myocarditis associated with COVID-19 vaccination: echocardiographic, cardiac tomography, and magnetic resonance imaging findings
- In-depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine
- Occurrence of acute infarct-like myocarditis after COVID-19 vaccination: just an accidental coincidence or rather a vaccination-associated autoimmune myocarditis?
- Recurrence of acute myocarditis temporally associated with receipt of coronavirus mRNA disease vaccine 2019 (COVID-19) in a male adolescent
- Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction?
- Self-limited myocarditis presenting with chest pain and ST-segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine
- Biopsy-proven lymphocytic myocarditis after the first COVID-19 mRNA vaccination in a 40-year-old man: case report
- Myocarditis and other cardiovascular complications of mRNA-based COVID-19 vaccines
- Case report: acute myocarditis after the second dose of SARS-CoV-2 mRNA-1273 vaccine mRNA-1273
- Acute myocardial infarction within 24 hours after COVID-19 vaccination.
- Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 COVID-19 mRNA vaccination in two patients.
- Lymphohistocytic myocarditis after vaccination with the COVID-19 viral vector Ad26.COV2.S
- Myocarditis associated with SARS-CoV-2 mRNA vaccination in children aged 12 to 17 years: stratified analysis of a national database
- A report of myocarditis adverse events in the U.S. Vaccine Adverse Event Reporting System (VAERS) in association with COVID-19 injectable biologics
- This study concludes that: “The vaccine was associated with an excess risk of myocarditis (1 to 5 events per 100,000 persons). The risk of this potentially serious adverse event and of many other serious adverse events increased substantially after SARS-CoV-2 infection”
- Myocarditis associated with SARS-CoV-2 mRNA vaccination in children aged 12 to 17 years: stratified analysis of a national database.
- Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents after co-vaccination
- Myocarditis after 2019 coronavirus disease mRNA vaccine: a case series and determination of incidence rate.
- Myocarditis and pericarditis after COVID-19 vaccination: inequalities in age and vaccine types.
- Epidemiology and clinical features of myocarditis/pericarditis before the introduction of COVID-19 mRNA vaccine in Korean children: a multicenter study.
- Shedding light on post-vaccination myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients.
- Myocarditis Following mRNA COVID-19 Vaccine
- Myocarditis following BNT162b2 mRNA Covid-19 mRNA vaccine in Israel.
- Myocarditis, pericarditis, and cardiomyopathy following COVID-19 vaccination.
- Myocarditis and other cardiovascular complications of COVID-19 mRNA-based COVID-19 vaccines.
- Possible Association Between COVID-19 Vaccine and Myocarditis: Clinical and CMR Findings.
- Hypersensitivity Myocarditis and COVID-19 Vaccines.
- Severe myocarditis associated with COVID-19 vaccine: zebra or unicorn?.
- Acute myocardial infarction and myocarditis after COVID-19 vaccination.
- Myocarditis after Covid-19 vaccination in a large healthcare organization.
- Association of myocarditis with COVID-19 messenger RNA BNT162b2 vaccine in a case series of children.
- Clinical suspicion of myocarditis temporally related to COVID-19 vaccination in adolescents and young adults.
- STEMI mimicry: focal myocarditis in an adolescent patient after COVID-19 mRNA vaccination.
- Myocarditis and pericarditis in association with COVID-19 mRNA vaccination: cases from a regional pharmacovigilance center.
- Myocarditis after COVID-19 mRNA vaccines.
- Patients with acute myocarditis after COVID-19 mRNA vaccination.
- Myocarditis after COVID-19 vaccination: a case series.
- Myocarditis associated with COVID-19 vaccination in adolescents.
- Myocarditis findings on cardiac magnetic resonance imaging after vaccination with COVID-19 mRNA in adolescents.
- Myocarditis after COVID-19 vaccination: magnetic resonance imaging study
- Acute myocarditis after administration of the second dose of BNT162b2 COVID-19 vaccine.
- Myocarditis after COVID-19 vaccination.
- Case report: probable myocarditis after Covid-19 mRNA vaccine in a patient with arrhythmogenic left ventricular cardiomyopathy.
- Acute myocarditis after administration of BNT162b2 vaccine against COVID-19.
- Myocarditis associated with COVID-19 mRNA vaccination.
- Acute myocarditis after COVID-19 vaccination: a case report
- Acute myopericarditis after COVID-19 vaccination in adolescents.
- Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19 vaccination.
- Acute myocarditis associated with anti-COVID-19 vaccination.
- Myocarditis associated with COVID-19 vaccination: echocardiographic, cardiac CT, and MRI findings.
- Acute symptomatic myocarditis in 7 adolescents after Pfizer-BioNTech COVID-19 vaccination.
- Myocarditis and pericarditis in adolescents after First and second doses of COVID-19 mRNA vaccines
- COVID 19 vaccine for adolescents. Concern for myocarditis and pericarditis.
- Cardiac imaging of acute myocarditis after vaccination with COVID-19 mRNA
- Myocarditis temporally associated with COVID-19 vaccination
- Acute myocarditis associated with COVID-19 vaccination: report of a case
- Myocarditis following vaccination with COVID-19 messenger RNA: a Japanese case series.
- Myocarditis in the setting of a recent COVID-19 vaccination.
- Acute myocarditis after a second dose of COVID-19 mRNA vaccine: report of two cases.
- Prevalence of thrombocytopenia, antiplatelet factor 4 antibodies, and elevated D-dimer in Thai people after vaccination with ChAdOx1 nCoV-19
- Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents after co-vaccination
- Myocarditis after 2019 coronavirus disease mRNA vaccine: a case series and incidence rate determination.
- Myocarditis and pericarditis after COVID-19 vaccination: inequalities in age and vaccine types
- Epidemiology and clinical features of myocarditis/pericarditis before the introduction of COVID-19 mRNA vaccine in Korean children
- Shedding light on post-vaccination myocarditis and pericarditis in COVID-19 and non-COVID-19 vaccine recipients
- Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents after co-vaccination
- Myocarditis-induced sudden death after BNT162b2 COVID-19 mRNA vaccination in Korea: case report focusing on histopathological findings
- Acute myocarditis after vaccination with COVID-19 mRNA in adults aged 18 years or older
- Recurrence of acute myocarditis temporally associated with receipt of the 2019 coronavirus mRNA disease vaccine (COVID-19) in an adolescent male
- Young male with myocarditis after mRNA-1273 coronavirus disease-2019 (COVID-19) mRNA vaccination
- Acute myocarditis after SARS-CoV-2 vaccination in a 24-year-old male
- Ga-DOTATOC digital PET images of inflammatory cell infiltrates in myocarditis after vaccination with COVID-19
- Occurrence of acute infarct-like myocarditis after vaccination with COVID-19: just an accidental coincidence or rather a vaccination-associated autoimmune myocarditis?
- Self-limited myocarditis presenting with chest pain and ST-segment elevation in adolescents after vaccination with BNT162b2 mRNA vaccine
- Myocarditis Following Immunization with COVID-19 mRNA Vaccines in Members of the U.S. Military
- Myocarditis after BNT162b2 vaccination in a healthy male
- Acute myocarditis after SARS-CoV-2 mRNA-1273 mRNA vaccination
- Biopsy-proven lymphocytic myocarditis after the first vaccination with COVID-19 mRNA in a 40-year-old man: case report
- Multimodality imaging and histopathology in a young man presenting with fulminant lymphocytic myocarditis and cardiogenic shock after vaccination with mRNA-1273
- Acute myocarditis after Comirnaty vaccination in a healthy male with previous SARS-CoV-2 infection
- Acute myocarditis in a young adult two days after vaccination with Pfizer
- Case report: acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus vaccination in 2019 requiring extracorporeal cardiopulmonary resuscitation
- Acute myocarditis after 2019 coronavirus disease vaccination
- A series of patients with myocarditis after vaccination against SARS-CoV-2 with mRNA-1279 and BNT162b2
- Acute myocarditis defined after vaccination with 2019 mRNA of coronavirus disease
- Biventricular systolic dysfunction in acute myocarditis after SARS-CoV-2 mRNA-1273 vaccination
- Myocarditis following COVID-19 vaccination: MRI study
- Acute myocarditis after COVID-19 vaccination: case report
- Association of myocarditis with COVID-19 messenger RNA BNT162b2 vaccine COVID-19 in a case series of children
- Clinical suspicion of myocarditis temporally related to COVID-19 vaccination in adolescents and young adults
- Myocarditis following vaccination with Covid-19 in a large healthcare organization
- Myocarditis and pericarditis in adolescents after the first and second doses of COVID-19 mRNA vaccines
- Myocarditis after SARS-CoV-2 mRNA vaccination, a case series
- Epidemiology of myocarditis and pericarditis following mRNA vaccines in Ontario, Canada: by vaccine product, schedule, and interval
- Acute myocardial infarction and myocarditis after COVID-19 vaccination
- Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients – Abbate, A., Gavin, J., Madanchi, N., Kim, C., Shah, P. R., Klein, K., . . . Danielides, S. (2021). Int J Cardiol, 340, 119-121.
- Myocarditis following COVID-19 mRNA vaccination – Abu Mouch, S., Roguin, A., Hellou, E., Ishai, A., Shoshan, U., Mahamid, L., . . . Berar Yanay, N. (2021). Vaccine, 39(29), 3790-3793.
- Myocarditis following COVID-19 vaccination – Albert, E., Aurigemma, G., Saucedo, J., & Gerson, D. S. (2021). Radiol Case Rep, 16(8), 2142-2145.
- Acute Myocardial Infarction and Myocarditis following COVID-19 Vaccination – Aye, Y. N., Mai, A. S., Zhang, A., Lim, O. Z. H., Lin, N., Ng, C. H., . . . Chew, N. W. S. (2021). QJM.
- STEMI Mimic: Focal Myocarditis in an Adolescent Patient After mRNA COVID-19 Vaccine – Azir, M., Inman, B., Webb, J., & Tannenbaum, L. (2021). J Emerg Med, 61(6), e129-e132.
- Myocarditis With COVID-19 mRNA Vaccines – Bozkurt, B., Kamat, I., & Hotez, P. J. (2021). Circulation, 144(6), 471-484.
- COVID 19 Vaccine for Adolescents. Concern about Myocarditis and Pericarditis – Calcaterra, G., Mehta, J. L., de Gregorio, C., Butera, G., Neroni, P., Fanos, V., & Bassareo, P. P. (2021). Pediatr Rep, 13(3), 530-533.
- Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? – Chamling, B., Vehof, V., Drakos, S., Weil, M., Stalling, P., Vahlhaus, C., . . . Yilmaz, A. (2021). Clin Res Cardiol, 110(11), 1850-1854.
- Cardiac MRI Findings of Myocarditis After COVID-19 mRNA Vaccination in Adolescents – Chelala, L., Jeudy, J., Hossain, R., Rosenthal, G., Pietris, N., & White, C. (2021). AJR Am J Roentgenol.
- Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings – Choi, S., Lee, S., Seo, J. W., Kim, M. J., Jeon, Y. H., Park, J. H., . . . Yeo, N. S. (2021). J Korean Med Sci, 36(40), e286.
- Epidemiology of Acute Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty Vaccination – Chua, G. T., Kwan, M. Y. W., Chui, C. S. L., Smith, R. D., Cheung, E. C., Tian, T., . . . Ip, P. (2021). Clin Infect Dis.
- Should T2 mapping be used in cases of recurrent myocarditis to differentiate between acute inflammation and chronic scar? – Clarke, R., & Ioannou, A. (2021). J Pediatr.
- Myocarditis and Pericarditis Following mRNA COVID-19 Vaccination: What Do We Know So Far? – Das, B. B., Moskowitz, W. B., Taylor, M. B., & Palmer, A. (2021). Children (Basel), 8(7).
- A Series of Patients With Myocarditis Following SARS-CoV-2 Vaccination With mRNA-1279 and BNT162b2 – Dickey, J. B., Albert, E., Badr, M., Laraja, K. M., Sena, L. M., Gerson, D. S., . . . Aurigemma, G. P. (2021). JACC Cardiovasc Imaging, 14(9), 1862-1863.
- Biopsy-proven lymphocytic myocarditis following thefirst mRNA COVID-19 vaccination in a 40-year-old male: case report – Ehrlich, P., Klingel, K., Ohlmann-Knafo, S., Huttinger, S., Sood, N., Pickuth, D., & Kindermann, M. (2021). Clin Res Cardiol, 110(11), 1855-1859.
- Acute myocarditis in a young adult two days after Pfizer vaccination – Facetti, S., Giraldi, M., Vecchi, A. L., Rogiani, S., & Nassiacos, D. (2021). G Ital Cardiol (Rome), 22(11), 891-893.
- Myocarditis and Pericarditis in Adolescents after First and Second doses of mRNA COVID-19 Vaccines – Foltran, D., Delmas, C., Flumian, C., De Paoli, P., Salvo, F., Gautier, S., . . . Montastruc, F. (2021). Eur Heart J Qual Care Clin Outcomes.
- Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices – United States, June 2021 – Gargano, J. W., Wallace, M., Hadler, S. C., Langley, G., Su, J. R., Oster, M. E., . . . Oliver, S. E. (2021). MMWR Morb Mortal Wkly Rep, 70(27), 977-982.
- A Late Presentation of COVID-19 Vaccine-Induced Myocarditis – Gautam, N., Saluja, P., Fudim, M., Jambhekar, K., Pandey, T., & Al’Aref, S. (2021). Cureus, 13(9), e17890.
- Myocarditis after vaccination against COVID-19 – Gellad, W. F. (2021). BMJ, 375, n3090.
- Myocarditis with the Pfizer/BioNTech and Moderna COVID-19 vaccines – In brief: Myocarditis with the Pfizer/BioNTech and Moderna COVID-19 vaccines. (2021). Med Lett Drugs Ther, 63(1629), e9.
- Myocarditis should be considered in those with a troponin rise and unobstructed coronary arteries following Pfizer-BioNTech COVID-19 vaccination – Ioannou, A. (2021a). QJM. doi:10.1093/qjmed/hcab231.
- T2 mapping should be utilized in cases of suspected myocarditis to confirm an acute inflammatory process – Ioannou, A. (2021b). QJM. doi:10.1093/qjmed/hcab326.
- Myocarditis Following COVID-19 Vaccination – Isaak, A., Feisst, A., & Luetkens, J. A. (2021). Radiology, 301(1), E378-E379. doi:10.1148/radiol.2021211766.
- Myocarditis and pericarditis in association with COVID-19 mRNA-vaccination: cases from a regional pharmacovigilance centre – Istampoulouoglou, I., Dimitriou, G., Spani, S., Christ, A., Zimmermanns, B., Koechlin, S., . . . Leuppi-Taegtmeyer, A. B. (2021). Glob Cardiol Sci Pract, 2021(3), e202118. doi:10.21542/gcsp.2021.18.
- COVID-19 Vaccination-Associated Myocarditis in Adolescents – Jain, S. S., Steele, J. M., Fonseca, B., Huang, S., Shah, S., Maskatia, S. A., . . . Grosse-Wortmann, L. (2021). Pediatrics, 148(5). doi:10.1542/peds.2021-053427.
- Young Male With Myocarditis Following mRNA-1273 Vaccination Against Coronavirus Disease-2019 (COVID-19) – Kaneta, K., Yokoi, K., Jojima, K., Kotooka, N., & Node, K. (2021). Circ J. doi:10.1253/circj.CJ-21-0818.
- Myocarditis following COVID-19 vaccination – Kaul, R., Sreenivasan, J., Goel, A., Malik, A., Bandyopadhyay, D., Jin, C., . . . Panza, J. A. (2021). Int J Cardiol Heart Vasc, 36, 100872. doi:10.1016/j.ijcha.2021.100872.
- Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination – Kim, H. W., Jenista, E. R., Wendell, D. C., Azevedo, C. F., Campbell, M. J., Darty, S. N., . . . Kim, R. J. (2021). JAMA Cardiol, 6(10), 1196-1201. doi:10.1001/jamacardio.2021.2828.
- Cardiac Imaging of Acute Myocarditis Following COVID-19 mRNA Vaccination – Kim, I. C., Kim, H., Lee, H. J., Kim, J. Y., & Kim, J. Y. (2021). J Korean Med Sci, 36(32), e229. doi:10.3346/jkms.2021.36.e229.
- Myocarditis following mRNA vaccination against SARS-CoV-2, a case series – King, W. W., Petersen, M. R., Matar, R. M., Budweg, J. B., Cuervo Pardo, L., & Petersen, J. W. (2021). Am Heart J Plus, 8, 100042. doi:10.1016/j.ahjo.2021.100042.
- mRNA COVID vaccine and myocarditis in adolescents – Kwan, M. Y. W., Chua, G. T., Chow, C. B., Tsao, S. S. L., To, K. K. W., Yuen, K. Y., . . . Ip, P. (2021). Hong Kong Med J, 27(5), 326-327. doi:10.12809/hkmj215120.
- Reply to “Letter to the editor: Myocarditis should be considered in those with a troponin rise and unobstructed coronary arteries following Pfizer-BioNTech COVID-19 vaccination” – Lee, E., Chew, N. W. S., Ng, P., & Yeo, T. J. (2021). QJM. doi:10.1093/qjmed/hcab232.
- Myocarditis following COVID-19 vaccination – A case series – Levin, D., Shimon, G., Fadlon-Derai, M., Gershovitz, L., Shovali, A., Sebbag, A., . . . Gordon, B. (2021). Vaccine, 39(42), 6195-6200. doi:10.1016/j.vaccine.2021.09.004.
- Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types – Li, M., Yuan, J., Lv, G., Brown, J., Jiang, X., & Lu, Z. K. (2021). J Pers Med, 11(11). doi:10.3390/jpm11111106.
- Case Report: Acute Fulminant Myocarditis and Cardiogenic Shock After Messenger RNA Coronavirus Disease 2019 Vaccination Requiring Extracorporeal Cardiopulmonary Resuscitation – Lim, Y., Kim, M. C., Kim, K. H., Jeong, I. S., Cho, Y. S., Choi, Y. D., & Lee, J. E. (2021). Front Cardiovasc Med, 8, 758996. doi:10.3389/fcvm.2021.758996.
- Myocarditis and Pericarditis After COVID-19 mRNA Vaccination: Practical Considerations for Care Providers – Luk, A., Clarke, B., Dahdah, N., Ducharme, A., Krahn, A., McCrindle, B., . . . McDonald, M. (2021). Can J Cardiol, 37(10), 1629-1634. doi:10.1016/j.cjca.2021.08.001.
- Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel – Mevorach, D., Anis, E., Cedar, N., Bromberg, M., Haas, E. J., Nadir, E., . . . Alroy-Preis, S. (2021). N Engl J Med, 385(23), 2140-2149. doi:10.1056/NEJMoa2109730.
- Recurrence of Acute Myocarditis Temporally Associated with Receipt of the mRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a Male Adolescent – Minocha, P. K., Better, D., Singh, R. K., & Hoque, T. (2021). J Pediatr, 238, 321-323. doi:10.1016/j.jpeds.2021.06.035.
- Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military – Montgomery, J., Ryan, M., Engler, R., Hoffman, D., McClenathan, B., Collins, L., . . . Cooper, L. T., Jr. (2021). JAMA Cardiol, 6(10), 1202-1206. doi:10.1001/jamacardio.2021.2833.
- Myocarditis Following a COVID-19 Messenger RNA Vaccination: A Japanese Case Series – Murakami, Y., Shinohara, M., Oka, Y., Wada, R., Noike, R., Ohara, H., . . . Ikeda, T. (2021). Intern Med. doi:10.2169/internalmedicine.8731-21.
- Acute Myocarditis Associated with COVID-19 Vaccination: A Case Report – Nagasaka, T., Koitabashi, N., Ishibashi, Y., Aihara, K., Takama, N., Ohyama, Y., . . . Kaneko, Y. (2021). J Cardiol Cases. doi:10.1016/j.jccase.2021.11.006.
- Epidemiology and Clinical Features of Myocarditis/Pericarditis before the Introduction of mRNA COVID-19 Vaccine in Korean Children: a Multicenter Study – Park, H., Yun, K. W., Kim, K. R., Song, S. H., Ahn, B., Kim, D. R., . . . Kim, Y. J. (2021). J Korean Med Sci, 36(32), e232. doi:10.3346/jkms.2021.36.e232.
- Self-limited myocarditis presenting with chest pain and ST segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine – Park, J., Brekke, D. R., & Bratincsak, A. (2021). Cardiol Young, 1-doi:10.1017/S1047951121002547.
- Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: a case – Patel, Y. R., Louis, D. W., Atalay, M., Agarwal, S., & Shah, N. R. (2021). J Cardiovasc Magn Reson, 23(1), 101. doi:10.1186/s12968-021-00795-1.
- Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection – Patone, M., Mei, X. W., Handunnetthi, L., Dixon, S., Zaccardi, F., Shankar-Hari, M., . . . Hippisley-Cox, J. (2021). Nat Med. doi:10.1038/s41591-021-01630-1.
- Acute myocarditis following Comirnaty vaccination in a healthy man with previous SARS-CoV-2 infection – Patrignani, A., Schicchi, N., Calcagnoli, F., Falchetti, E., Ciampani, N., Argalia, G., & Mariani, A. (2021). Radiol Case Rep, 16(11), 3321-3325. doi:10.1016/j.radcr.2021.07.082.
- Myocarditis Following COVID-19 mRNA Vaccine: A Case Series and Incidence Rate Determination – Perez, Y., Levy, E. R., Joshi, A. Y., Virk, A., Rodriguez-Porcel, M., Johnson, M., . . . Swift, M. D. (2021). Clin Infect Dis. doi:10.1093/cid/ciab926.
- Myocarditis following COVID-19 vaccination: magnetic resonance imaging study – Shiyovich, A., Witberg, G., Aviv, Y., Eisen, A., Orvin, K., Wiessman, M., . . . Hamdan, A. (2021). Eur Heart J Cardiovasc Imaging. doi:10.1093/ehjci/jeab230.
- Acute Myocarditis Following COVID-19 mRNA Vaccination in Adults Aged 18 Years or Older – Simone, A., Herald, J., Chen, A., Gulati, N., Shen, A. Y., Lewin, B., & Lee, M. S. (2021). JAMA Intern Med, 181(12), 1668-1670. doi:10.1001/jamainternmed.2021.5511.
- Risk of Myocarditis from COVID-19 Infection in People Under Age 20: A Population-Based Analysis – Singer, M. E., Taub, I. B., & Kaelber, D. C. (2021). medRxiv. doi:10.1101/2021.07.23.21260998.
- Myocarditis Associated with mRNA COVID-19 Vaccination – Starekova, J., Bluemke, D. A., Bradham, W. S., Grist, T. M., Schiebler, M. L., & Reeder, S. B. (2021). Radiology, 301(2), E409-E411. doi:10.1148/radiol.2021211430.
- Temporal association between the COVID-19 Ad26.COV2.S vaccine and acute myocarditis: A case report and literature review – Sulemankhil, I., Abdelrahman, M., & Negi, S. I. (2021). Cardiovasc Revasc Med. doi:10.1016/j.carrev.2021.08.012.
- Case report: acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine – Tailor, P. D., Feighery, A. M., El-Sabawi, B., & Prasad, A. (2021). Eur Heart J Case Rep, 5(8), ytab319. doi:10.1093/ehjcr/ytab319.
- Eosinophilic Myocarditis Following Coronavirus Disease 2019 (COVID-19) Vaccination – Takeda, M., Ishio, N., Shoji, T., Mori, N., Matsumoto, M., & Shikama, N. (2021). Circ J. doi:10.1253/circj.CJ-21-935.
- Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults – Truong, D. T., Dionne, A., Muniz, J. C., McHugh, K. E., Portman, M. A., Lambert, L. M., . . . Newburger, J. W. (2021). Circulation. doi:10.1161/CIRCULATIONAHA.121.056583.
- Myocarditis and Other Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines – Vidula, M. K., Ambrose, M., Glassberg, H., Chokshi, N., Chen, T., Ferrari, V. A., & Han, Y. (2021). Cureus, 13(6), e15576. doi:10.7759/cureus.15576.
- Myocarditis Following mRNA COVID-19 Vaccine – Visclosky, T., Theyyunni, N., Klekowski, N., & Bradin, S. (2021). Pediatr Emerg Care, 37(11), 583-584. doi:10.1097/PEC.0000000000002557.
- Myocarditis after BNT162b2 vaccination in a healthy male – Watkins, K., Gri몭 n, G., Septaric, K., & Simon, E. L. (2021). Am J Emerg Med, 50, 815 e811-815 e812. doi:10.1016/j.ajem.2021.06.051.
- Myocarditis after Covid-19 Vaccination in a Large Health Care Organization – Witberg, G., Barda, N., Hoss, S., Richter, I., Wiessman, M., Aviv, Y., . . . Kornowski, R. (2021). N Engl J Med, 385(23), 2132-2139. doi:10.1056/NEJMoa2110737.
- Myocarditis with the Pfizer/BioNTech and Moderna COVID-19 vaccines (2021). Med Lett Drugs Ther, 63(1629), e9. Retrieved from PubMed
- Myocarditis should be considered in those with a troponin rise and unobstructed coronary arteries following Pfizer-BioNTech COVID-19 vaccination. QJM. doi:10.1093/qjmed/hcab231. Author: Ioannou, A.
- Myocarditis Following COVID-19 Vaccination. Radiology, 301(1), E378-E379. doi:10.1148/radiol.2021211766. Authors: Isaak, A., Feisst, A., & Luetkens, J. A.
- Myocarditis and pericarditis in association with COVID-19 mRNA-vaccination: cases from a regional pharmacovigilance centre. Glob Cardiol Sci Pract, 2021(3), e202118. doi:10.21542/gcsp.2021.18. Authors: Istampoulouoglou, I., Dimitriou, G., Spani, S., Christ, A., Zimmermanns, B., Koechlin, S., . . . Leuppi-Taegtmeyer, A. B.
- COVID-19 Vaccination-Associated Myocarditis in Adolescents. Pediatrics, 148(5). doi:10.1542/peds.2021-053427. Authors: Jain, S. S., Steele, J. M., Fonseca, B., Huang, S., Shah, S., Maskatia, S. A., . . . Grosse-Wortmann, L.
- Young Male With Myocarditis Following mRNA-1273 Vaccination Against Coronavirus Disease-2019 (COVID-19). Circ J. doi:10.1253/circj.CJ-21-0818. Authors: Kaneta, K., Yokoi, K., Jojima, K., Kotooka, N., & Node, K.
- Myocarditis following COVID-19 vaccination. Int J Cardiol Heart Vasc, 36, 100872. doi:10.1016/j.ijcha.2021.100872. Authors: Kaul, R., Sreenivasan, J., Goel, A., Malik, A., Bandyopadhyay, D., Jin, C., . . . Panza, J. A.
- Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol, 6(10), 1196-1201. doi:10.1001/jamacardio.2021.2828. Authors: Kim, H. W., Jenista, E. R., Wendell, D. C., Azevedo, C. F., Campbell, M. J., Darty, S. N., . . . Kim, R. J.
- Cardiac Imaging of Acute Myocarditis Following COVID-19 mRNA Vaccination. J Korean Med Sci, 36(32), e229.doi:10.3346/jkms.2021.36.e229. Authors: Kim, I. C., Kim, H., Lee, H. J., Kim, J. Y., & Kim, J. Y.
- Myocarditis following mRNA vaccination against SARS-CoV-2, a case series. Am Heart J Plus, 8, 100042. doi:10.1016/j.ahjo.2021.100042. Authors: King, W. W., Petersen, M. R., Matar, R. M., Budweg, J. B., Cuervo Pardo, L., & Petersen, J. W.
- mRNA COVID vaccine and myocarditis in adolescents. Hong Kong Med J, 27(5), 326-327. doi:10.12809/hkmj215120. Authors: Kwan, M. Y. W., Chua, G. T., Chow, C. B., Tsao, S. S. L., To, K. K. W., Yuen, K. Y., . . . Ip, P.
- Reply to “Letter to the editor: Myocarditis should be considered in those with a troponin rise and unobstructed coronary arteries following PfizerBioNTech COVID-19 vaccination”. QJM.doi:10.1093/qjmed/hcab232. Authors: Lee, E., Chew, N. W. S., Ng, P., & Yeo, T. J.
- Myocarditis following COVID-19 vaccination – A case series. Vaccine, 39(42), 6195-6200.doi:10.1016/j.vaccine.2021.09.004. Authors: Levin, D., Shimon, G., Fadlon-Derai, M., Gershovitz, L., Shovali, A., Sebbag, A., . . . Gordon, B.
- Myocarditis and Pericarditis following COVID-19 Vaccination: Inequalities in Age and Vaccine Types. J Pers Med, 11(11). doi:10.3390/jpm11111106. Authors: Li, M., Yuan, J., Lv, G., Brown, J., Jiang, X., & Lu, Z. K.
- Acute Fulminant Myocarditis and Cardiogenic Shock After Messenger RNA Coronavirus Disease 2019 Vaccination Requiring Extracorporeal Cardiopulmonary Resuscitation. Front Cardiovasc Med, 8, 758996. doi:10.3389/fcvm.2021.758996. Authors: Lim, Y., Kim, M. C., Kim, K. H., Jeong, I. S., Cho, Y. S., Choi, Y. D., & Lee, J. E.
- Myocarditis and Pericarditis After COVID-19 mRNA Vaccination: Practical Considerations for Care Providers. Can J Cardiol, 37(10), 1629-1634.doi:10.1016/j.cjca.2021.08.001. Authors: Luk, A., Clarke, B., Dahdah, N., Ducharme, A., Krahn, A., McCrindle, B., . . . McDonald, M.
- Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med, 385(23), 2140-2149. doi:10.1056/NEJMoa2109730. Authors: Mevorach, D., Anis, E., Cedar, N., Bromberg, M., Haas, E. J., Nadir, E., . . . Alroy-Preis, S.
- Recurrence of Acute Myocarditis Temporally Associated with Receipt of the mRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a Male Adolescent. J Pediatr, 238, 321-323.doi:10.1016/j.jpeds.2021.06.035. Authors: Minocha, P. K., Better, D., Singh, R. K., & Hoque, T.
- Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol, 6(10), 1202-1206.doi:10.1001/jamacardio.2021.2833. Authors: Montgomery, J., Ryan, M., Engler, R., Hoffman, D., McClenathan, B., Collins, L., . . . Cooper, L. T., Jr.
- Myocarditis Following a COVID-19 Messenger RNA Vaccination: A Japanese Case Series. Intern Med. doi:10.2169/internalmedicine.8731-21. Authors: Murakami, Y., Shinohara, M., Oka, Y., Wada, R., Noike, R., Ohara, H., . . . Ikeda, T.
- Acute Myocarditis Associated with COVID-19 Vaccination: A Case Report. Authors: Nagasaka, T., Koitabashi, N., Ishibashi, Y., Aihara, K., Takama, N., Ohyama, Y., . . . Kaneko, Y. Published in J Cardiol Cases. doi:10.1016/j.jccase.2021.11.006.
- Untimely Myocardial Infarction or COVID-19 Vaccine Side Effect
- Acute Myocardial Infarction Within 24 Hours After COVID-19 Vaccination: Is Kounis Syndrome the Culprit?
- A Case of Acute Encephalopathy and Non-ST-Segment Elevation Myocardial Infarction after Vaccination with mRNA-1273: Possible Adverse Effect.
- Acute Myocardial Infarction within 24 Hours after COVID-19 Vaccination: Is Kounis Syndrome Clinical and Histopathologic Spectrum of Delayed Adverse Skin Reactions after COVID-19 Vaccination.
- Features of Inflammatory Heart Reactions Following mRNA COVID-19 Vaccination at a Global Level. Authors: Chouchana, L., Blet, A., Al-Khalaf, M., Kaffl, T. S., Nair, G., Robblee, J., . . . Liu, P. P. Published in Clin Pharmacol Ther. doi:10.1002/cpt.2499.
- Acute Fulminant Myocarditis and Cardiogenic Shock After Messenger RNA Coronavirus Disease 2019 Vaccination Requiring Extracorporeal Cardiopulmonary Resuscitation.
- Lymphohistocytic Myocarditis after Ad26.COV2.S Viral Vector COVID-19 Vaccination.
26 Myopericarditis
- Myopericarditis after Pfizer mRNA COVID-19 Vaccination in Adolescents
- Myopericarditis after Vaccination with COVID-19 mRNA in Adolescents 12 to 18 Years of Age
- Important Information on Myopericarditis after Vaccination with Pfizer COVID-19 mRNA in Adolescents
- Insights from a Murine Model of COVID-19 mRNA Vaccine-Induced Myopericarditis: Could Accidental Intravenous Injection of a Vaccine Induce Myopericarditis
- Acute Myocarditis after Administration of BNT162b2 Vaccine against COVID-19
- Insights from a Murine Model of Myopericarditis Induced by COVID-19 mRNA Vaccine: Could Accidental Intravenous Injection of a Vaccine Induce Myopericarditis
- COVID-19 mRNA Vaccination and Development of CMR-Confirmed Myopericarditis
- Intravenous Injection of Coronavirus Disease 2019 (COVID-19) mRNA Vaccine Can Induce Acute Myopericarditis in a Mouse Model
- Myopericarditis in a Previously Healthy Adolescent Male after COVID-19 Vaccination: Case Report
- Report of a Case of Myopericarditis after Vaccination with BNT162b2 COVID-19 mRNA in a Young Korean Male
- Myopericarditis after Pfizer Messenger Ribonucleic Acid Coronavirus Disease Vaccine in Adolescents
- Acute Myopericarditis after COVID-19 Vaccine in Adolescents
- Population-based Incidence of Myopericarditis After COVID-19 Vaccination in Danish Adolescents
- Myopericarditis After the Pfizer Messenger Ribonucleic Acid Coronavirus Disease Vaccine in Adolescents
- mRNA Coronavirus-19 Vaccine-Associated Myopericarditis in Adolescents: A Survey Study
- Important Insights into Myopericarditis after the Pfizer mRNA COVID-19 Vaccination in Adolescents
- Myopericarditis in a Previously Healthy Adolescent Male Following COVID-19 Vaccination: A Case Report
- Recurrence of Myopericarditis Following mRNA COVID-19 Vaccination in a Male Adolescent
- Myopericarditis After Messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18 Years of Age
- Influenza Vaccination and Myo-Pericarditis in Patients Receiving Immune Checkpoint Inhibitors
- Myopericarditis in a Previously Healthy Adolescent Male After COVID-19 Vaccination: Case Report
27 Nephrotic Syndrome references
- Nephrotic Syndrome After ChAdOx1 nCoV-19 Vaccine Against SARS-CoV-2
- New-Onset Nephrotic Syndrome After Janssen COVID-19 Vaccination: Case Report and Literature Review
- Hematuria, a Generalized Petechial Rash, and Headaches After Oxford AstraZeneca ChAdOx1 nCoV-19 Vaccination
- A Case of Outbreak of Macroscopic Hematuria and IgA Nephropathy After SARS-CoV-2 Vaccination
28 Neurological Symptoms references
- Neurological Symptoms and Neuroimaging Alterations Related to COVID-19 Vaccine: Cause or Coincidence
- Neurological Symptoms and Neuroimaging Alterations Related to COVID-19 Vaccine: Cause or Coincidence?
- Spectrum of Neurological Complications After COVID-19 Vaccination
- Non-hospital Observational Study of Neurological Disorders in Patients Recently Vaccinated with COVID-19 mRNA Vaccines
- Neurological Side Effects of SARS-CoV-2 Vaccines
- Neurological Complications After the First Dose of COVID-19 Vaccines and SARS-CoV-2 Infection
30 Pericarditis references
- Myocarditis and Pericarditis After Vaccination with COVID-19 mRNA: Practical Considerations for Care Providers
- Myocarditis, Pericarditis, and Cardiomyopathy After COVID-19 Vaccination
- Myocarditis and Pericarditis After COVID-19 Vaccination
- Pericarditis After Administration of BNT162b2 mRNA COVID-19 mRNA Vaccine
- Epidemiology and Clinical Features of Myocarditis/Pericarditis Before the Introduction of COVID-19 mRNA Vaccine in Korean Children: A Multicenter Study
- Myocarditis, Pericarditis, and Cardiomyopathy After COVID-19 Vaccination
- Clinical Guidance for Young People with Myocarditis and Pericarditis After Vaccination with COVID-19 mRNA
- Myocarditis/Pericarditis Associated with COVID-19 Vaccine
- Acute Myocarditis After the Second Dose of SARS-CoV-2 Vaccine: Serendipity or Causal Relationship
- Pericarditis After Administration of COVID-19 mRNA BNT162b2 Vaccine
- Unusual Presentation of Acute Pericarditis After Vaccination Against SARS-COV-2 mRNA-1237 Modern
- A Case Series of Acute Pericarditis After Vaccination with COVID-19 in the Context of Recent Reports from Europe and the United States
- Acute Pericarditis and Cardiac Tamponade After Vaccination with Covid-19
- Pericarditis After Administration of the BNT162b2 mRNA Vaccine COVID-19
- Case Report: Symptomatic Pericarditis Post COVID-19 Vaccination
31 Perimyocarditis references
- Perimyocarditis in Adolescents After Pfizer-BioNTech COVID-19 Vaccine
- Perimyocarditis in Adolescents After Pfizer-BioNTech COVID-19 Vaccine
- Unusual Presentation of Acute Perimyocarditis After Modern SARS-COV-2 mRNA-1237 Vaccination
- Perimyocarditis After the First Dose of mRNA-1273 SARS-CoV-2 (Moderna) Vaccine in a Young Healthy Male: Case Report
- Acute Perimyocarditis After the First Dose of COVID-19 mRNA Vaccine
- Perimyocarditis After Vaccination With COVID-19
- Tinoco, M., Leite, S., Faria, B., Cardoso, S., Von Hafe, P., Dias, G., . . . Lourenco, A. (2021). Perimyocarditis Following COVID-19 Vaccination. Clin Med Insights Cardiol, 15, 11795468211056634. doi:10.1177/11795468211056634.
- Jhaveri, R., Adler-Shohet, F. C., Blyth, C. C., Chiotos, K., Gerber, J. S., Green, M., . . . Zaoutis, T. (2021). Weighing the Risks of Perimyocarditis With the Benefits of SARS-CoV-2 mRNA Vaccination in Adolescents. J Pediatric Infect Dis Soc, 10(10), 937-939.doi:10.1093/jpids/piab061.
- Khogali, F., & Abdelrahman, R. (2021). Unusual Presentation of Acute Perimyocarditis Following SARS-COV-2 mRNA-1237 Moderna Vaccination. Cureus, 13(7), e16590.doi:10.7759/cureus.16590.
- Hasnie, A. A., Hasnie, U. A., Patel, N., Aziz, M. U., Xie, M., Lloyd, S. G., & Prabhu, S. D. (2021). Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc Disord, 21(1), 375. doi:10.1186/s12872-021-02183-3
34 Psoriasis references
- Onset/Outbreak of Psoriasis After Coronavirus ChAdOx1 nCoV-19 Vaccine (Oxford-AstraZeneca/Covishield): Report of Two Cases
- Exacerbation of Plaque Psoriasis After COVID-19 Inactivated mRNA and BNT162b2 Vaccines: Report of Two Cases
- Miller Fisher Syndrome After Pfizer COVID-19 Vaccine
- Miller Fisher Syndrome After 2019 BNT162b2 mRNA Coronavirus Vaccination
35 Pulmonary Embolism references
- Pulmonary Embolism, Transient Ischemic Attack, and Thrombocytopenia After Johnson & Johnson COVID-19 Vaccine
- A Case of Acute Pulmonary Embolism After Immunization with SARS-CoV-2 mRNA
- Isolated pulmonary embolism after COVID vaccination: 2 case reports and a review of acute pulmonary embolism complications and follow-up
- Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in persons aged 75 years or older
- Beware of neuromyelitis optica spectrum disorder after vaccination with inactivated virus for COVID-19
- Neuromyelitis optica in a healthy woman after vaccination against severe acute respiratory syndrome coronavirus 2 mRNA-1273
- Shingles-like skin lesion after vaccination with AstraZeneca for COVID-19: a case report
- Recurrent herpes zoster after COVID-19 vaccination in patients with chronic urticaria on cyclosporine treatment – A report of 3 cases
37 Rhabdomyolysis references
- Rhabdomyolysis and Fasciitis Induced by the COVID-19 mRNA Vaccine
- COVID-19 Vaccine-Induced Rhabdomyolysis: Case Report with Literature Review
- COVID-19 Vaccine-Induced Rhabdomyolysis: Case Report with Review of the Literature
- Rhabdomyolysis and Fasciitis Induced by COVID-19 mRNA Vaccine
- Case Report: ANCA-Associated Vasculitis Presenting with Rhabdomyolysis and Crescentic Pauci-Immune Glomerulonephritis After Vaccination with Pfizer-BioNTech COVID-19 mRNA
39 Takotsubo Cardiomyopathy references
- Myocarditis, Pericarditis, and Cardiomyopathy After COVID-19 Vaccination
- Takotsubo Cardiomyopathy After Vaccination with mRNA COVID-19
- Takotsubo (Stress) Cardiomyopathy After Vaccination with ChAdOx1 nCoV-19
- Takotsubo Cardiomyopathy After Coronavirus 2019 Vaccination in a Patient on Maintenance Hemodialysis
- Takotsubo Syndrome After COVID-19 Vaccination
40 Thrombocytopenia References
- Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death After ChAdOx1 nCoV-19 Vaccination.
- US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Vaccination with Ad26.COV2.S (against COVID-19), March 2 to April 21, 2020.
- Management of Cerebral and Splanchnic Vein Thrombosis Associated with Thrombocytopenia in Subjects Previously Vaccinated with Vaxzevria (AstraZeneca): Position Statement of the Italian Society for the Study of Hemostasis and Thrombosis (SISET).
- Vaccine-Induced Immune Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After Vaccination with COVID-19: A Systematic Review.
- Thrombosis with Thrombocytopenia Syndrome Associated with COVID-19 Vaccines.
- COVID-19 Vaccine-Induced Thrombosis and Thrombocytopenia: A Commentary on an Important and Practical Clinical Dilemma.
- Thrombosis with Thrombocytopenia Syndrome Associated with COVID-19 Viral Vector Vaccines.
- COVID-19 Vaccine-Induced Immune-Immune Thrombotic Thrombocytopenia: An Emerging Cause of Splanchnic Vein Thrombosis.
- The Roles of Platelets in COVID-19-Associated Coagulopathy and Vaccine-Induced Immune Thrombotic Immune Thrombocytopenia (COVID).
- Thrombotic Immune Thrombocytopenia Induced by SARS-CoV-2 Vaccine.
- Thrombosis and Thrombocytopenia After Vaccination with ChAdOx1 nCoV-19.
- Post-Mortem Findings in Vaccine-Induced Thrombotic Thrombocytopenia (COVID-19).
- Thrombocytopenia, Including Immune Thrombocytopenia After Receiving COVID-19 mRNA Vaccines Reported to the Vaccine Adverse Event Reporting System (VAERS).
- Hypothesis Behind the Very Rare Cases of Thrombosis with Thrombocytopenia Syndrome After SARS-CoV-2 Vaccination.
- Primary Adrenal Insufficiency Associated with Thrombotic Immune Thrombocytopenia Induced by the Oxford-AstraZeneca ChAdOx1 nCoV-19 Vaccine (VITT).
- Early Results of Bivalirudin Treatment for Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After Vaccination with Ad26.COV2.S.
- Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection.
- Vaccine-Induced Thrombotic Thrombocytopenia: The Dark Chapter of a Success Story.
- Cerebral Venous Sinus Thrombosis and Thrombocytopenia After COVID-19 Vaccination: Report of Two Cases in the United Kingdom.
- Immune Thrombocytopenic Purpura After Vaccination with COVID-19 Vaccine (ChAdOx1 nCoV-19).
- Vaccine-Induced Thrombotic Thrombocytopenia, a Rare but Severe Case of Friendly Fire in the Battle Against the COVID-19 Pandemic: What Pathogenesis?
- Thrombocytopenia and Intracranial Venous Sinus Thrombosis After Exposure to the “AstraZeneca COVID-19 Vaccine”
- Thrombocytopenia Following Pfizer and Moderna SARS-CoV-2 Vaccination
- Severe and Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2 Vaccination
- Purpuric Rash and Thrombocytopenia After mRNA-1273 (Moderna) COVID-19 Vaccine
- Thrombosis with Thrombocytopenia After Messenger RNA Vaccine-1273
- First Dose of ChAdOx1 and BNT162b2 COVID-19 Vaccines and Thrombocytopenic, Thromboembolic, and Hemorrhagic Events in Scotland
- Exacerbation of Immune Thrombocytopenia After COVID-19 Vaccination
- PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia
- Antibody Epitopes in Vaccine-Induced Immune Thrombotic Thrombocytopenia
- Thrombosis with Thrombocytopenia Syndrome Associated with COVID-19 Vaccines
- Immune Thrombocytopenia Associated with Pfizer-BioNTech’s COVID-19 BNT162b2 mRNA Vaccine
- Immune Thrombosis and Thrombocytopenia (VITT) Associated with the COVID-19 Vaccine: Diagnostic and Therapeutic Recommendations for a New Syndrome
- Laboratory Testing for Suspicion of COVID-19 Vaccine-Induced Thrombotic (Immune) Thrombocytopenia
- Intracerebral Haemorrhage Due to Thrombosis with Thrombocytopenia Syndrome After COVID-19 Vaccination: The First Fatal Case in Korea
- Risk of Thrombocytopenia and Thromboembolism After COVID-19 Vaccination and Positive SARS-CoV-2 Tests: Self-Controlled Case Series Study
- Vaccine-Induced Immune Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination: A Systematic Review
- Primary Adrenal Insufficiency Associated with Thrombotic Immune Thrombocytopenia Induced by Oxford-AstraZeneca ChAdOx1 nCoV-19 Vaccine (VITT)
- Cerebral Venous Thrombosis and Vaccine-Induced Thrombocytopenia.a. Oxford-AstraZeneca COVID-19: A Missed Opportunity for a Rapid Return on Experience
- Immune Thrombocytopenia in a 22-Year-Old Post COVID-19 Vaccine
- Secondary Immune Thrombocytopenia (ITP) Associated with ChAdOx1 COVID-19 Vaccine: Case Report
- Thrombosis with Thrombocytopenia Syndrome (TTS) Following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 Vaccination: Risk-Benefit Analysis for Persons <60 Years in Australia
- Bilateral Superior Ophthalmic Vein Thrombosis, Ischemic Stroke, and Immune Thrombocytopenia After Vaccination with ChAdOx1 nCoV-19
- Risk of Thrombocytopenia and Thromboembolism After COVID-19 Vaccination and Positive SARS-CoV-2 Tests: Self-Controlled Case Series Study
- First Dose of ChAdOx1 and BNT162b2 COVID-19 Vaccines and Thrombocytopenic, Thromboembolic, and Hemorrhagic Events in Scotland
- Thrombocytopenia After COVID-19 Vaccination
- A Case of Multiple Thrombocytopenia and Thrombosis Following Vaccination with ChAdOx1 nCoV-19 Against SARS-CoV-2
- Vaccine-Induced Thrombotic Thrombocytopenia: The Elusive Link Between Thrombosis and Adenovirus-Based SARS-CoV-2 Vaccines
- Acute Ischemic Stroke Revealing Immune Thrombotic Thrombocytopenia Induced by ChAdOx1 nCov-19 Vaccine: Impact on Recanalization Strategy
- Procoagulant Antibody-Mediated Procoagulant Platelets in Immune Thrombotic Thrombocytopenia Associated with SARS-CoV-2 Vaccination
- Thrombotic Thrombocytopenia After Vaccination with COVID-19: In Search of the Underlying Mechanism
- Thrombosis and SARS-CoV-2 Vaccines: Vaccine-Induced Immune Thrombotic Thrombocytopenia
- Acquired Thrombotic Thrombocytopenic Thrombocytopenic Purpura: A Rare Disease Associated with the BNT162b2 Vaccine
- Immune Complexes, Innate Immunity and NETosis in ChAdOx1 Vaccine-Induced Thrombocytopenia
- Immune-Mediated Thrombocytopenic Purpura After Pfizer-BioNTech COVID-19 Vaccine in an Elderly Woman
- Immune-Mediated Thrombocytopenia Associated with Ad26.COV2.S Vaccine (Janssen; Johnson & Johnson)
- Transient Thrombocytopenia with Glycoprotein-Specific Platelet Autoantibodies After Vaccination with Ad26.COV2.S: Case Report
- COVID-19 Vaccine, Immune Thrombotic Thrombocytopenia, Jaundice, Hyperviscosity: Concern in Cases with Underlying Hepatic Problems
- Immune Thrombocytopenia After Vaccination During the COVID-19 Pandemic
- Vaccine-Induced Thrombocytopenia with Severe Headache
- Vaccine-Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis with a High Mortality Rate: A Case Series
- Adenovirus Interactions with Platelets and Coagulation and Vaccine-Associated Autoimmune Thrombocytopenia Thrombosis Syndrome
- Australian and New Zealand Approach to the Diagnosis and Treatment of Vaccine-Induced Immune Thrombosis and Immune Thrombocytopenia
- An Observational Study to Identify the Prevalence of Thrombocytopenia and Anti-PF4 / Polyanion Antibodies in Norwegian Health Care Workers After COVID-19 Vaccination
- A Rare Case of Thrombosis and Thrombocytopenia of the Superior Ophthalmic Vein After ChAdOx1 nCoV-19 Vaccination Against SARS-CoV-2
- Thrombosis and Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines: Vaccine-Induced Immune Thrombotic Thrombocytopenia
- Renal Vein Thrombosis and Pulmonary Embolism Secondary to Vaccine-Induced Thrombotic Immune Thrombocytopenia (VITT)
- Limb Ischemia and Pulmonary Artery Thrombosis After ChAdOx1 nCoV-19 Vaccine (Oxford-AstraZeneca): A Case of Vaccine-Induced Immune Thrombotic Thrombocytopenia
- A Case of Vaccine-Induced Immune-Immune Thrombotic Thrombocytopenia with Massive Arteriovenous Thrombosis
- Thrombocytopenia in an Adolescent with Sickle Cell Anemia After COVID-19 Vaccination
- Vaccine-Induced Thrombocytopenia with Severe Headache
- ChAdOx1 Interacts with CAR and PF4 with Implications for Thrombosis with Thrombocytopenia Syndrome
- Lethal Vaccine-Induced Immune Thrombotic Immune Thrombocytopenia (VITT) Following Announcement 26.COV2.S: First Documented Case Outside the U.S.
- A Prothrombotic Thrombocytopenic Disorder Resembling Heparin-Induced Thrombocytopenia After Coronavirus-19 Vaccination
- VITT (Vaccine-Induced Immune Thrombotic Thrombocytopenia) After Vaccination with ChAdOx1 nCoV-19
- Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT): A New Clinicopathologic Entity with Heterogeneous Clinical Presentations
- Treatment of Acute Ischemic Stroke Associated with ChAdOx1 nCoV-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia
- Cerebral Venous Sinus Thrombosis After Vaccination: The UK Experience
- Cerebral Venous Vein/Venous Sinus Thrombosis with Thrombocytopenia Syndrome After COVID-19 Vaccination
- Portal Vein Thrombosis Due to Vaccine-Induced Immune Thrombotic Immune Thrombocytopenia (VITT) After COVID Vaccination with ChAdOx1 nCoV-19
- Spontaneous HIT Syndrome: Knee Replacement, Infection, and Parallels with Vaccine-Induced Immune Thrombotic Thrombocytopenia
- Thrombocytopenia with Acute Ischemic Stroke and Hemorrhage in a Patient Recently Vaccinated with an Adenoviral Vector-Based COVID-19 Vaccine
- ChAdOx1 nCoV-19 Vaccine-Associated Thrombocytopenia: Three Cases of Immune Thrombocytopenia After 107,720 Doses of ChAdOx1 Vaccination in Thailand
- Pulmonary Embolism, Transient Ischemic Attack, and Thrombocytopenia After Johnson & Johnson COVID-19 Vaccine
- Neurosurgical Considerations with Respect to Decompressive Craniectomy for Intracerebral Hemorrhage After SARS-CoV-2 Vaccination in Vaccine-Induced Thrombotic Thrombocytopenia-VITT
- Secondary Thrombocytopenia After SARS-CoV-2 Vaccination: Case Report of Hemorrhage and Hematoma After Minor Oral Surgery
- Venous Thromboembolism and Mild Thrombocytopenia After Vaccination with ChAdOx1 nCoV-19
- Fatal Exacerbation of ChAdOx1-nCoV-19-Induced Thrombotic Thrombocytopenia Syndrome After Successful Initial Therapy with Intravenous Immunoglobulins: A Rationale for Monitoring Immunoglobulin G Levels
- A Rare Case of COVID-19 Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Affecting the Venosplanchnic and Pulmonary Arterial Circulation from a UK District General Hospital
- COVID-19 Vaccine-Induced Thrombotic Thrombocytopenia: A Case Series
- Immune Thrombocytopenia After Immunization with Vaxzevria ChAdOx1-S Vaccine (AstraZeneca), Victoria, Australia
- Case Report of Immune Thrombocytopenia After Vaccination with ChAdOx1 nCoV-19
- Thrombocytopenia with Acute Ischemic Stroke and Hemorrhage in a Patient Recently Vaccinated with an Adenoviral Vector-Based COVID-19 Vaccine
- Intracerebral Hemorrhage and Thrombocytopenia After AstraZeneca COVID-19 Vaccine: Clinical and Diagnostic Challenges of Vaccine-Induced Thrombotic Thrombocytopenia
- Thrombocytopenia, Including Immune Thrombocytopenia After Receiving COVID-19 mRNA Vaccines Reported to the Vaccine Adverse Event Reporting System (VAERS)
- Newly Diagnosed Immune Thrombocytopenia in a Pregnant Patient After Coronavirus Disease 2019 Vaccination
- Thrombocytopenia in an Adolescent with Sickle Cell Anemia After COVID-19 Vaccination
- Abdominal Pain and Bilateral Adrenal Hemorrhage from Immune Thrombotic Thrombocytopenia Induced by COVID-19 Vaccine
- Venous Thromboembolism and Mild Thrombocytopenia After ChAdOx1 nCoV-19 Vaccination
- Severe Immune Thrombocytopenia Following COVID-19 Vaccination: Report of Four Cases and Review of the Literature
- Relapse of Immune Thrombocytopenia After COVID-19 Vaccination
- Images of Immune Thrombotic Thrombocytopenia Induced by Oxford/AstraZeneca® COVID-19 Vaccine
- Adenovirus Interactions with Platelets and Coagulation and Vaccine-Induced Immune Thrombotic Thrombocytopenia Syndrome
- Complicated Case Report of Long-Term Vaccine-Induced Thrombotic Immune Thrombocytopenia A
- Prevalence of Thrombocytopenia, Anti-Platelet Factor 4 Antibodies, and Elevated D-Dimer in Thai people After Vaccination with ChAdOx1 nCoV-19
- Bilateral Thalamic Stroke: A Case of COVID-19 (VITT) Vaccine-Induced Immune Thrombotic Thrombocytopenia or a Coincidence Due to Underlying Risk Factors
- Successful Treatment of Vaccine-Induced Immune Thrombotic Thrombocytopenia in a 26-Year-Old Female Patient
- Case Report: Vaccine-Induced Immune Thrombotic Thrombocytopenia in a Patient with Pancreatic Cancer After Vaccination with Messenger RNA-1273
- Vaccine-Induced Thrombotic Thrombocytopenia After Ad26.COV2.S Vaccination in a Man Presenting as Acute Venous Thromboembolism
- Severe, Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2 Vaccine
- Risk of Thrombocytopenia and Thromboembolism After COVID-19 Vaccination and SARS-CoV-2 Positive Testing: Self-Controlled Case Series Study
- Thrombocytopenia Following Pfizer and Moderna SARS-CoV-2 Vaccination
- Thrombocytopenia Including Immune Thrombocytopenia After Receipt of mRNA COVID-19 Vaccines Reported to the Vaccine Adverse Event Reporting System (VAERS)
- Relapse of Immune Thrombocytopenia After COVID-19 Vaccination in Young Male Patient
- Peduncular, Symptomatic Cavernous Bleeding After Immune Thrombocytopenia-Induced SARS-CoV-2 Vaccination
- Vaccine-Induced Immune Thrombotic Thrombocytopenia with Disseminated Intravascular Coagulation and Death Following the ChAdOx1 nCoV-19 Vaccine
41 Thrombosis references
- Three Cases of Acute Venous Thromboembolism in Women After Vaccination Against COVID-19.
- Acute Coronary Tree Thrombosis After Vaccination for COVID-19.
- US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia After Vaccination with Ad26.COV2.S (Against COVID-19), March 2 to April 21, 2020.
- Portal Vein Thrombosis Associated with ChAdOx1 nCov-19 Vaccine.
- Management of Cerebral and Splanchnic Vein Thrombosis Associated with Thrombocytopenia in Subjects Previously Vaccinated with Vaxzevria (AstraZeneca): Position Statement of the Italian Society for the Study of Hemostasis and Thrombosis (SISET).
- Thrombosis With Thrombocytopenia Syndrome Associated With COVID-19 Vaccines.
- COVID-19 Vaccine-Induced Thrombosis and Thrombocytopenia: A Commentary on an Important and Practical Clinical Dilemma.
- Thrombosis With Thrombocytopenia Syndrome Associated With COVID-19 Viral Vector Vaccines.
- COVID-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia: An Emerging Cause of Splanchnic Vein Thrombosis.
- The Roles of Platelets in COVID-19-Associated Coagulopathy and Vaccine-Induced Immune Thrombotic Immune Thrombocytopenia (COVID).
- Roots of Autoimmunity of Thrombotic Events After COVID-19 Vaccination.
- Thrombotic Immune Thrombocytopenia Induced by SARS-CoV-2 Vaccine.
- Thrombosis and Thrombocytopenia After Vaccination with ChAdOx1 nCoV-19.
- Thrombotic Thrombocytopenia After Vaccination with ChAdOx1 nCov-19.
- Post-Mortem Findings in Vaccine-Induced Thrombotic Thrombocytopenia (COVID-19).
- Comparison of Vaccine-Induced Thrombotic Episodes Between ChAdOx1 nCoV-19 and Ad26.COV.2.S Vaccines.
- Hypothesis Behind the Very Rare Cases of Thrombosis With Thrombocytopenia Syndrome After SARS-CoV-2 Vaccination.
- Primary Adrenal Insufficiency Associated With Thrombotic Immune Thrombocytopenia Induced by the Oxford-AstraZeneca ChAdOx1 nCoV-19 Vaccine (VITT).
- Portal Vein Thrombosis Occurring After the First Dose of SARS-CoV-2 mRNA Vaccine in a Patient With Antiphospholipid Syndrome.
- Early Results of Bivalirudin Treatment for Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After Vaccination With Ad26.COV2.S.
- Mechanisms of Immunothrombosis in Vaccine-Induced Thrombotic Thrombocytopenia (VITT) Compared to Natural SARS-CoV-2 Infection.
- Prothrombotic Immune Thrombocytopenia After COVID-19 Vaccination.
- Vaccine-Induced Thrombotic Thrombocytopenia: The Dark Chapter of a Success Story.
- Thrombosis After COVID-19 Vaccination: Possible Link to ACE Pathways.
- Vaccine-Induced Thrombotic Thrombocytopenia, a Rare but Severe Case of Friendly Fire in the Battle Against the COVID-19 Pandemic: What Pathogenesis?.
- Thrombocytopenia and Intracranial Venous Sinus Thrombosis After Exposure to the “AstraZeneca COVID-19 Vaccine”.
- Thrombosis With Thrombocytopenia After Messenger RNA Vaccine-1273.
- First Dose of ChAdOx1 and BNT162b2 COVID-19 Vaccines and Thrombocytopenic, Thromboembolic, and Hemorrhagic Events in Scotland.
- PF4 Immunoassays in Vaccine-Induced Thrombotic Thrombocytopenia.
- Antibody Epitopes in Vaccine-Induced Immune Immune Thrombotic Thrombocytopenia.
- Thrombosis With Thrombocytopenia Syndrome Associated With COVID-19 Vaccines.
- Immune Thrombosis and Thrombocytopenia (VITT) Associated With the COVID-19 Vaccine: Diagnostic and Therapeutic Recommendations for a New Syndrome.
- Laboratory Testing for Suspicion of COVID-19 Vaccine-Induced Thrombotic (Immune) Thrombocytopenia.
- Intracerebral Haemorrhage Due to Thrombosis With Thrombocytopenia Syndrome After COVID-19 Vaccination: The First Fatal Case in Korea.
- Risk of Thrombocytopenia and Thromboembolism After COVID-19 Vaccination and Positive SARS-CoV-2 Tests: Self-Controlled Case Series Study.
- Vaccine-Induced Immune Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination; A Systematic Review.
- Primary Adrenal Insufficiency Associated With Thrombotic Immune Thrombocytopenia Induced by Oxford-AstraZeneca ChAdOx1 nCoV-19 Vaccine (VITT).
- Thromboaspiration Infusion and Fibrinolysis for Portomesenteric Thrombosis After Administration of AstraZeneca COVID-19 Vaccine.
- 59-Year-Old Woman With Extensive Deep Venous Thrombosis and Pulmonary Thromboembolism 7 Days After a First Dose of Pfizer-BioNTech BNT162b2 mRNA Vaccine COVID-19.
- Thrombosis With Thrombocytopenia Syndrome (TTS) Following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 Vaccination: Risk-Benefit Analysis for Persons <60 Years in Australia.
- Comparison of Vaccine-Induced Thrombotic Events Between ChAdOx1 nCoV-19 and Ad26.COV.2.S Vaccines.
- Bilateral Superior Ophthalmic Vein Thrombosis, Ischemic Stroke and Immune Thrombocytopenia After Vaccination With ChAdOx1 nCoV-19.
- Celiac Artery and Splenic Artery Thrombosis Complicated by Splenic Infarction 7 Days After the First Dose of Oxford Vaccine, Causal Relationship or Coincidence.
- Primary Adrenal Insufficiency Associated With Oxford-AstraZeneca ChAdOx1 nCoV-19 (VITT) Vaccine-Induced Immune Thrombotic Thrombocytopenia.
- Thrombosis With Thrombocytopenia Syndrome After COVID-19 Immunization.
- Thrombosis With Thrombocytopenia Syndrome Associated With COVID-19 Viral Vector Vaccines.
- Thromboaspiration Infusion and Fibrinolysis for Portomesenteric Thrombosis After Administration of the AstraZeneca COVID-19 Vaccine.
- Atypical Thrombosis Associated With the Vaccine VaxZevria® (AstraZeneca): Data From the French Network of Regional Pharmacovigilance Centers.
- Vaccine-Induced Thrombosis and Thrombocytopenia With Bilateral Adrenal Hemorrhage.
- Palmar Digital Vein Thrombosis After Oxford-AstraZeneca COVID-19 Vaccination.
- Cutaneous Thrombosis Associated With Cutaneous Necrosis Following Oxford-AstraZeneca COVID-19 Vaccination.
- Thrombosis With Thrombocytopenia After Messenger Vaccine RNA-1273.
- Coronavirus (COVID-19) Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT).
- Comparison of Adverse Drug Reactions Among Four COVID-19 Vaccines in Europe Using the EudraVigilance Database: Thrombosis in Unusual Sites.
- Immunoglobulin Adjuvant for Vaccine-Induced Immune Thrombotic Thrombocytopenia.
- Severe Vaccine-Induced Thrombotic Thrombocytopenia Following Vaccination With COVID-19: An Autopsy Case Report and Review of the Literature.
- Platelet Activation and Modulation in Thrombosis With Thrombocytopenia Syndrome Associated With the ChAdO × 1 nCov-19 Vaccine.
- Report of the International Cerebral Venous Thrombosis Consortium on Cerebral Venous Thrombosis After SARS-CoV-2 Vaccination.
- Immune Thrombocytopenia Associated With the Pfizer-BioNTech COVID-19 mRNA Vaccine BNT162b2.
- Secondary Immune Thrombocytopenia Putatively Attributable to COVID-19 Vaccination.
- Immune Thrombocytopenia Following Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine.
- Newly Diagnosed Idiopathic Thrombocytopenia After COVID-19 Vaccine Administration.
- Idiopathic Thrombocytopenic Purpura and the Modern COVID-19 Vaccine.
- Thrombocytopenia After Pfizer and Moderna SARS-CoV-2 Vaccination.
- Immune Thrombocytopenic Purpura and Acute Liver Injury After COVID-19 Vaccination.
- Carotid Artery Immune Thrombosis Induced by Adenovirus-Vectored COVID-19 Vaccine: Case Report.
- The Roles of Platelets in COVID-19-Associated Coagulopathy and Vaccine-Induced Immune-Thrombotic Thrombocytopenia.
- Cerebral Venous Sinus Thrombosis Negative for Anti-PF4 Antibody Without Thrombocytopenia After Immunization With COVID-19 Vaccine in a Non-Comorbid Elderly Indian Male Treated With Conventional Heparin-Warfarin-Based Anticoagulation.
- Arterial Events, Venous Thromboembolism, Thrombocytopenia, and Bleeding After Vaccination With Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population-Based Cohort Study.
- Procoagulant Microparticles: A Possible Link Between Vaccine-Induced Immune Thrombocytopenia (VITT) and Cerebral Sinus Venous Thrombosis.
- U.S. Case Reports of Cerebral Venous Sinus Thrombosis With Thrombocytopenia After Vaccination With Ad26.COV2.S, March 2-April 21, 2021.
- Malignant Cerebral Infarction After Vaccination With ChAdOx1 nCov-19: A Catastrophic Variant of Vaccine-Induced Immune-Mediated Thrombotic Thrombocytopenia.
- Acute Ischemic Stroke Revealing Immune Thrombotic Thrombocytopenia Induced by ChAdOx1 nCov-19 Vaccine: Impact on Recanalization Strategy.
- Vaccine-Induced Immune Thrombotic Immune Thrombocytopenia (VITT): A New Clinicopathologic Entity With Heterogeneous Clinical Presentations.
- Imaging and Hematologic Findings in Thrombosis and Thrombocytopenia After Vaccination With ChAdOx1 nCoV-19 (AstraZeneca).
- Autoimmunity Roots of Thrombotic Events After Vaccination With COVID-19.
- Cerebral Venous Sinus Thrombosis After Vaccination: The UK Experience.
- Cutaneous Thrombosis Associated With Cutaneous Necrosis Following Oxford-AstraZeneca COVID-19 Vaccination.
- Myocardial Infarction and Azygos Vein Thrombosis After Vaccination With ChAdOx1 nCoV-19 in a Hemodialysis Patient.
- Refractory Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) Treated With Delayed Therapeutic Plasma Exchange (TPE).
- Rare Case of COVID-19 Vaccine-Associated Intracranial Hemorrhage With Venous Sinus Thrombosis.
- Delayed Headache After COVID-19 Vaccination: A Warning Sign for Vaccine-Induced Cerebral Venous Thrombosis.
- Clinical Features of Vaccine-Induced Thrombocytopenia and Immune Thrombosis.
- Predictors of Mortality in Thrombotic Thrombocytopenia After Adenoviral COVID-19 Vaccination: The FAPIC Score.
- Ischemic Stroke as a Presenting Feature of Immune Thrombotic Thrombocytopenia Induced by ChAdOx1-nCoV-19 Vaccination.
- Endovascular Treatment for Vaccine-Induced Cerebral Venous Sinus Thrombosis and Thrombocytopenia After Vaccination with ChAdOx1 nCoV-19: Report of Three Cases.
- Possible Triggers of Thrombocytopenia and/or Hemorrhage by BNT162b2 Vaccine, Pfizer-BioNTech.
- Multiple Sites of Arterial Thrombosis in a 35-Year-Old Patient After Vaccination With ChAdOx1 (AstraZeneca), Which Required Emergency Femoral and Carotid Surgical Thrombectomy.
- Case Series of Vaccine-Induced Thrombotic Thrombocytopenia in a London Teaching Hospital.
- Neuro-Ophthalmic Complications With Thrombocytopenia and Thrombosis Induced by ChAdOx1 nCoV-19 Vaccine.
- Thrombotic Events After COVID-19 Vaccination in Over 50 Years of Age: Results of a Population-Based Study in Italy.
- Intracerebral Hemorrhage Associated With Vaccine-Induced Thrombotic Thrombocytopenia After ChAdOx1 nCOVID-19 Vaccination in a Pregnant Woman.
- Age- and Sex-Specific Incidence of Cerebral Venous Sinus Thrombosis Associated With Ad26.COV2.S COVID-19 Vaccination.
- Genital Necrosis With Cutaneous Thrombosis Following Vaccination With COVID-19 mRNA.
- Cerebral Venous Sinus Thrombosis After mRNA-Based COVID-19 Vaccination.
- COVID-19 Vaccine-Induced Immune Thrombosis With Thrombocytopenia Thrombosis (VITT) and Shades of Gray in Thrombus Formation.
- Acute ST-Segment Elevation Myocardial Infarction Secondary to Vaccine-Induced Immune Thrombosis With Thrombocytopenia (VITT).
- Thrombosis With Thrombocytopenia Syndrome (TTS) After Vaccination With AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19: A Risk-Benefit Analysis for Persons <60 Years in Australia.
- Characteristics and Outcomes of Patients With Cerebral Venous Sinus Thrombosis in Thrombotic Immune Thrombocytopenia Induced by SARS-CoV-2 Vaccine.
- Case Study of Thrombosis and Thrombocytopenia Syndrome After Administration of the AstraZeneca COVID-19 Vaccine.
- Thrombosis with Thrombocytopenia Syndrome Associated with COVID-19 Vaccines.
- Cerebral Venous Sinus Thrombosis Following Vaccination with ChAdOx1: The First Case of Definite Thrombosis with Thrombocytopenia Syndrome in India.
- COVID-19 Vaccine-Associated Thrombosis with Thrombocytopenia Syndrome (TTS): Systematic Review and Post Hoc Analysis.
- Concerns for Adverse Effects of Thrombocytopenia and Thrombosis After Adenovirus-Vectored COVID-19 Vaccination.
- Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination: Neurologic and Radiologic Management.
- Case Report: Cerebral Sinus Vein Thrombosis in Two Patients with AstraZeneca SARS-CoV-2 Vaccine.
- Vaccine-Induced Immune Thrombosis and Thrombocytopenia Syndrome After Adenovirus-Vectored Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination: A New Hypothesis on Mechanisms and Implications for Future Vaccine Development.
- Thrombosis in Peripheral Artery Disease and Thrombotic Thrombocytopenia Following Adenoviral COVID-19 Vaccination.
- Cerebral Venous Sinus Thrombosis and Thrombotic Events After Vector-Based COVID-19 Vaccines: Systematic Review and Meta-Analysis.
- Thrombosis After COVID-19 Vaccination: Possible Link to ACE Pathways.
- Major Artery Thrombosis and Vaccination Against ChAdOx1 nCov-19.
- Understanding the Risk of Thrombosis with Thrombocytopenia Syndrome Following Ad26.COV2.S Vaccination.
- Comments on Thrombosis After Vaccination: Spike Protein Leader Sequence Could Be Responsible for Thrombosis and Antibody-Mediated Thrombocytopenia.
- Thrombosis in Pre- and Post-Vaccination Phase of COVID-19.
- Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Combined Venous Micro-Macrothrombosis.
- Thrombosis and Thrombocytopenia Syndrome Causing Isolated Symptomatic Carotid Occlusion After COVID-19 Ad26.COV2.S Vaccine (Janssen).
- An Unusual Presentation of Acute Deep Vein Thrombosis After Modern COVID-19 Vaccine: Case Report.
- Immediate High-Dose Intravenous Immunoglobulins Followed by Direct Treatment With Thrombin Inhibitors Is Crucial for Survival in Vaccine-Induced Immune Thrombotic Thrombocytopenia SARS-Covid-19-Vector Adenoviral VITT With Venous Thrombosis of the Cerebral Sinus and Portal Vein.
- Thrombosis Formation After COVID-19 Vaccination Immunologic Aspects: Review Article.
- Imaging and Hematologic Findings in Thrombosis and Thrombocytopenia After Vaccination With ChAdOx1 nCoV-19 (AstraZeneca).
- Cerebral Venous Sinus Thrombosis, Pulmonary Embolism, and Thrombocytopenia After COVID-19 Vaccination in a Taiwanese Man: A Case Report and Review of the Literature.
- Fatal Cerebral Venous Sinus Thrombosis After COVID-19 Vaccination.
- Autoimmune Roots of Thrombotic Events After COVID-19 Vaccination.
- New Portal Vein Thrombosis in Cirrhosis: Is Thrombophilia Exacerbated by Vaccine or COVID-19.
- Cerebral Venous Sinus Thrombosis After Vaccination With COVID-19 mRNA of BNT162b2.
- A Case of Unusual Mild Clinical Presentation of COVID-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia With Splanchnic Vein Thrombosis.
- Cerebral Venous Sinus Thrombosis Following Vaccination With Pfizer-BioNTech COVID-19 (BNT162b2).
- A Case of Idiopathic Thrombocytopenic Purpura After a Booster Dose of COVID-19 BNT162b2 Vaccine (Pfizer-Biontech).
- Vaccine-Induced Immune Thrombotic Immune Thrombocytopenia (VITT): Targeting Pathologic Mechanisms With Bruton’s Tyrosine Kinase Inhibitors.
- Thromboembolic Events in Younger Females Exposed to Pfizer-BioNTech or Moderna COVID-19 Vaccines.
- Thrombosis After Adenovirus-Vectored COVID-19 Vaccination: A Concern for Underlying Disease.
- Unusual Site of Deep Vein Thrombosis After Vaccination Against Coronavirus mRNA-2019 Coronavirus Disease (COVID-19).
- Deep Venous Thrombosis After Vaccination With Ad26.COV2.S in Adult Males.
- Clinical and Biological Features of Cerebral Venous Sinus Thrombosis After Vaccination With ChAdOx1 nCov-19.
- COV2-S Vaccination May Reveal Hereditary Thrombophilia: Massive Cerebral Venous Sinus Thrombosis in a Young Man With Normal Platelet Count.
- Post-mortem Findings in Vaccine-Induced Thrombotic Thrombocytopenia.
- COVID-19 Vaccine-Induced Thrombosis.
- Inflammation and Platelet Activation After COVID-19 Vaccines: Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis.
- Anaphylactoid Reaction and Coronary Thrombosis Related to COVID-19 mRNA Vaccine.
- Occurrence of Splenic Infarction Due to Arterial Thrombosis After Vaccination With COVID-19.
- Deep Venous Thrombosis More Than Two Weeks After COVID-19 Vaccination.
- Information on ChAdOx1 nCoV-19 Vaccine-Induced Immune-Mediated Thrombotic Thrombocytopenia.
- Management of a Patient With a Rare Congenital Limb Malformation Syndrome After SARS-CoV-2 Vaccine-Induced Thrombosis and Thrombocytopenia (VITT).
- Thrombocytopenia and Splanchnic Thrombosis After Vaccination With Ad26.COV2.S Successfully Treated With Transjugular Intrahepatic Portosystemic Shunt and Thrombectomy.
- Chang, J. C., & Hawley, H. B. (2021). Vaccine-Associated Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to Venous Combined Micro-Macrothrombosis. Medicina (Kaunas), 57(11). doi:10.3390/medicina57111163.
- Potential Risk of Thrombotic Events After COVID-19 Vaccination With Oxford-AstraZeneca in Women Receiving Estrogen.
- Thrombotic Adverse Events Reported for Moderna, Pfizer, and Oxford-AstraZeneca COVID-19 Vaccines: Comparison of Occurrence and Clinical Outcomes in the EudraVigilance Database.
- Predicted and Observed Incidence of Thromboembolic Events Among Koreans Vaccinated With the ChAdOx1 nCoV-19 Vaccine.
- Three Cases of Acute Venous Thromboembolism in Women After Coronavirus 2019 Vaccination.
- Shazley, O., & Alshazley, M. (2021). A COVID-Positive 52-Year-Old Man Presented With Venous Thromboembolism and Disseminated Intravascular Coagulation Following Johnson & Johnson Vaccination: A Case-Study. Cureus, 13(7), e16383.doi:10.7759/cureus.16383.
42 Thrombotic Thrombocytopenic Purpura references
- Thrombotic Thrombocytopenic Purpura after Vaccination with Ad26.COV2-S
- Thrombotic Thrombocytopenic Purpura: A New Threat after COVID BNT162b2 Vaccine
- Severe Immune Thrombocytopenic Purpura after SARS-CoV-2 Vaccine
- Immune Thrombocytopenic Purpura Associated with COVID-19 mRNA Vaccine Pfizer-BioNTech BNT16B2b2
43 Vasculitis References
- ANCA-Associated Vasculitis After Pfizer-BioNTech COVID-19 Vaccine
- Propylthiouracil-Induced Neutrophil Anti-Cytoplasmic Antibody-Associated Vasculitis After COVID-19 Vaccination
- IgA Vasculitis in Adult Patient After Vaccination with ChAdOx1 nCoV-19
- A Case of Leukocytoclastic Vasculitis After Vaccination with a SARS-CoV2 Vaccine: Case Report
- A Case of ANCA-Associated Vasculitis After AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 Vaccination: Victim or Causality?
- Leukocytoclastic Vasculitis as a Cutaneous Manifestation of ChAdOx1 Corona Virus Vaccine nCoV-19 (Recombinant)
- Induction of Cutaneous Leukocytoclastic Vasculitis After ChAdOx1 nCoV-19 Vaccine
- Recurrent ANCA-Associated Vasculitis After Oxford AstraZeneca ChAdOx1-S COVID-19 Vaccination: A Case Series of Two Patients
- Cutaneous Lymphocytic Vasculitis After Administration of the Second Dose of AZD1222 (Oxford-AstraZeneca) Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine: Chance or Causality
- Case Report: Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis with Acute Renal Failure and Pulmonary Hemorrhage Can Occur After COVID-19 Vaccination
- Intracerebral Hemorrhage Due to Vasculitis Following COVID-19 Vaccination: Case Report
- COVID-19 Vaccine-Induced Urticarial Vasculitis
- ANCA-Associated Vasculitis After Pfizer-BioNTech COVID-19 Vaccine
- New-Onset Leukocytoclastic Vasculitis After COVID-19 Vaccine
- Cutaneous Small Vessel Vasculitis After COVID-19 Vaccine
- Outbreak of Leukocytoclastic Vasculitis After COVID-19 Vaccine
- Leukocytoclastic Vasculitis After Exposure to COVID-19 Vaccine
- Vasculitis and Bursitis in [18F] FDG-PET/CT After COVID-19 mRNA Vaccine: Post Hoc Ergo Propter Hoc?
- Cutaneous Lymphocytic Vasculitis After Administration of COVID-19 mRNA Vaccine
- Cutaneous Leukocytoclastic Vasculitis Induced by Sinovac COVID-19 Vaccine
- Reactivation of IgA Vasculitis After Vaccination with COVID-19
- Varicella-Zoster Virus-Related Small-Vessel Vasculitis After Pfizer-BioNTech COVID-19 Vaccination
- Imaging in Vascular Medicine: Leukocytoclastic Vasculitis After COVID-19 Vaccine Booster
- Cutaneous Vasculitis Following COVID-19 Vaccination
- Possible Case of COVID-19 mRNA Vaccine-Induced Small-Vessel Vasculitis
- IgA Vasculitis Following COVID-19 Vaccination in an Adult
- Propylthiouracil-Induced Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis Following Vaccination with COVID-19
- Coronavirus Disease Vaccine 2019 (COVID-19) in Systemic Lupus Erythematosus and Neutrophil Anti-Cytoplasmic Antibody-Associated Vasculitis
- Reactivation of IgA Vasculitis After COVID-19 Vaccination
- First Description of Immune Complex Vasculitis After COVID-19 Vaccination with BNT162b2: Case Report
- Nephrotic Syndrome and Vasculitis After SARS-CoV-2 Vaccine: True Association or Circumstantial
- Occurrence of De Novo Cutaneous Vasculitis After Vaccination Against Coronavirus Disease (COVID-19)
- Asymmetric Cutaneous Vasculitis After COVID-19 Vaccination with Unusual Preponderance of Eosinophils
- Granulomatous Vasculitis After AstraZeneca Anti-SARS-CoV-2 Vaccine
- A Case of Generalized Sweet’s Syndrome with Vasculitis Triggered by Recent Vaccination with COVID-19
- Small-Vessel Vasculitis Following Oxford-AstraZeneca Vaccination Against SARS-CoV-19
- Cutaneous Vasculitis After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine
- Leukocytoclastic Vasculitis After Coronavirus Disease Vaccination 2019
- Outbreaks of Mixed Cryoglobulinemia Vasculitis After Vaccination Against SARS-CoV-2
- Cutaneous Small-Vessel Vasculitis After Vaccination with a Single Dose of Janssen Ad26.COV2.S
- Case of Immunoglobulin A Vasculitis After Vaccination Against Coronavirus Disease 2019
- Relapse of Microscopic Polyangiitis After COVID-19 Vaccination: Case Report

Evidence that the Spike Protein is Toxic
MECHANISMS OF HARM CAUSED BY THE “SPIKE PROTEIN”
- Avolio E et al., “The SARS-CoV-2 Spike Protein Disrupts Human Cardiac Pericytes Function through CD147 Receptor-Mediated Signalling: A Potential Non-infective Mechanism of COVID-19 Microvascular Disease,” Clinical Science 135, no. 24. (December 22, 2021): 2667–2689, doi: https://doi.org/10.1042/CS20210735
- Biering SB et al., “SARS-CoV-2 Spike Triggers Barrier Dysfunction and Vascular Leak via Integrins and TGF-β Signaling,” Nature Communications 13 (2022): 7630, https://doi.org.10.1038/s41467-022-34910-5
- Boschi C et al., “SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Efects,” International Journal of Biological Macromolecules 23, no. 24 (2022): 15480, doi: https://doi.org/10.3390/ijms232415480
- Fontes-Dantas FL, “SARS-CoV-2 Spike Protein Induces TLR4-Mediated Long- Term Cognitive Dysfunction Recapitulating Post-COVID-19 Syndrome in Mice,” Cell Reports 42, no. 3 (March 2023):112189, doi: https://doi.org/10.1016/j.celrep.2023.112189 https://pubmed.ncbi.nlm.nih.gov/36857178/
- Frank MG et al., “SARS-CoV-2 Spike S1 Subunit Induces Neuroinflammatory, Microglial and Behavioral Sickness Responses: Evidence of PAMP-Like Properties,” Brain, Behavior, and Immunity 100 (February 2022): 267277, doi: https://doi.org/10.1016/j.bbi.2021.12.007
- Grobbelaar LM et al., “SARS-CoV-2 Spike Protein S1 Induces Fibrin(ogen) Resistant to Fibrinolysis: Implications for Microclot Formation in COVID-19,” Biosicence Reports 41, no. 8 (August 27, 2021): BSR20210611, doi: https://doi.org/10.1042/BSR20210611
- Idrees D and Vijay Kumar, “SARS-CoV-2 Spike Protein Interactions with Amyloidogenic Proteins: Potential Clues to Neurodegeneration,” Biochemical and Biophysical Research Communications 554 : 94–98, doi: https://doi.org/10.1016/j.bbrc.2021.03.100
- Khan S et al., “SARS-CoV-2 Spike Protein Induces Inflammation via TLR2-Dependent Activation of the NF-κB Pathway,” eLife 10 (December 6, 2021): e68563, doi: https://doi.org/10.7554/elife.68563
- Lei Y et al., “SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2,” Circulation Research 128, no. 9 (2021): 1323–1326, doi: https://doi.org/10.1161/CIRCRESAHA.121.318902
- Nuovo JG et al., “Endothelial Cell Damage Is the Central Part of COVID-19 and a Mouse Model Induced by Injection of the S1 Subunit of the Spike Protein.” Ann. Diagn. Pathol. 2021, 51, 151682. doi: https://doi.org/10.1016/j.anndiagpath.2020.151682
- Parry PL et al., “‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA,” Biomedicine 11, no. 8 (August 17, 2023): 2287, doi: https://doi.org/10.3390/biomedicines11082287
- Patra T et al., “SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells.” PLoS Pathog. 2020;16:e1009128. doi: https://doi.org/10.1371/journal.ppat.1009128
https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1009128
- Raghavan S et al., “SARS-CoV-2 Spike Protein Induces Degradation of Junctional Proteins That Maintain Endothelial Barrier Integrity.” Front. Cardiovasc. Med. 2021, 8, 687783. doi: https://doi.org/10.3389/fcvm.2021.687783
- Robles JP et al., “The Spike Protein of SARS-CoV-2 Induces Endothelial Inflammation through Integrin α5β1 and NF-κB Signaling,” JBC 298, no. 3 (March 2022):3, 101695, https://doi.org.10.1016/j.jbc.2022.101695
- Shirato K and Takako Kizaki, “SARS-CoV-2 Spike Protein S1 Subunit Induces Pro- inflammatory Responses via Toll-Like Receptor 4 Signaling in Murine and Human Macrophages,” Heliyon 7, no. 2 (February 2, 2021):e06187, doi: https://doi.org/10.1016/j.heliyon.2021.e06187
- Singh N and Anuradha Bharara Singh, “S2 Subunit of SARS-nCoV-2 Interacts with Tumor Suppressor Protein p53 and BRCA: An in Silico Study,” Translational Oncology 13, no. 10 (October 2020): 100814, doi: https://doi.org/10.1016/j.tranon.2020.100814
- Sui Y et al., “SARS-CoV-2 Spike Protein Suppresses ACE2 and Type I Interferon Expression in Primary Cells From Macaque Lung Bronchoalveolar Lavage,” Frontiers in Immunology 12 (June 4, 2021), https://doi.org.10.3389/fimmu.2021.658428
- Zhao Y et al., “SARS-CoV-2 spike protein interacts with and activates TLR4.” Cell Res. 2021;31:818–820. doi: https://doi.org/10.1038/s41422-021-00495-9
- Zheng Y et al., “SARS-CoV-2 Spike Protein Causes Blood Coagulation and Thrombosis by Competitive Binding to Heparan Sulfate,” International Journal of Biological Macromolecules 193 (December 15, 2021): 1124– 1129, doi: https://doi.org/10.1016/j.ijbiomac.2021.10.112


 KC’s COVID FactsScientific Studies on Vaccine InjuriesThis post mainly consists of scientific studies (both peer reviewed and preprints), systematic reviews, case studies (a few key ones of the thousands out there), and other medical journal articles that support the assertions in my Vaccine Injury post from November 2022. I began bookmarking these studies in late 2021, but since I’ve surely missed some, t…Read more5 months ago · 601 likes · 233 comments · KC
KC’s COVID FactsScientific Studies on Vaccine InjuriesThis post mainly consists of scientific studies (both peer reviewed and preprints), systematic reviews, case studies (a few key ones of the thousands out there), and other medical journal articles that support the assertions in my Vaccine Injury post from November 2022. I began bookmarking these studies in late 2021, but since I’ve surely missed some, t…Read more5 months ago · 601 likes · 233 comments · KC
Why would the World Health Organization publish the guide below if the COVID-19 mRNA injections were “safe?”

https://iris.who.int/bitstream/handle/10665/366581/9789240069206-eng.pdf

https://retractionwatch.com/retracted-coronavirus-covid-19-papers/

Articles by Children’s Health Defense
https://childrenshealthdefense.org/defender/peer-reviewed-study-moratorium-covid-mrna-vaccines/
https://childrenshealthdefense.org/defender/guillain-barre-syndrome-covid-flu-vaccines/
https://childrenshealthdefense.org/defender/covid-vaccine-side-effects-german-survey/
https://childrenshealthdefense.org/defender/astrazeneca-covid-vaccine-liability-ruling/
https://childrenshealthdefense.org/defender/teen-brains-aged-faster-during-lockdowns-worse-girls/
https://childrenshealthdefense.org/defender/fda-covid-vaccine-injury-emails-chd-foia/
https://childrenshealthdefense.org/defender/astrazeneca-dismiss-covid-vaccine-trial-injury-lawsuit/
https://childrenshealthdefense.org/defender/covid-vaccine-injury-largest-payout-claims-pending-cicp/
https://childrenshealthdefense.org/defender/trent-lieffring-student-died-after-pfizer-covid-19-shot/
https://childrenshealthdefense.org/defender/nih-covid-vaccine-injury-chd-foia-pharma-liability/
https://childrenshealthdefense.org/defender/spike-protein-five-mechanisms-damage-human-body/
https://childrenshealthdefense.org/defender/fauci-ignored-covid-vaccine-injuries-emails-chd/
https://childrenshealthdefense.org/defender/william-donald-judah-hospital-protocol-death-covid/
https://childrenshealthdefense.org/defender/cdc-redact-myocarditis-information-foia-covid-shots/
https://childrenshealthdefense.org/defender/defender-in-depth-john-stockton-rick-jaffe-free-speech/
https://childrenshealthdefense.org/defender/drugmakers-prioritize-profits-covid-senate-roundtable/
https://childrenshealthdefense.org/defender/brianne-dressen-covid-vaccine-injured/
https://childrenshealthdefense.org/defender/john-springer-covid-remdesivir-ventilator-death/
https://childrenshealthdefense.org/defender/defender-in-depth-podcast-albert-benavides/
https://childrenshealthdefense.org/defender/mathew-crawford-excess-deaths-defender-in-depth/
https://childrenshealthdefense.org/defender/lawsuits-uk-astrazeneca-covid-vaccine-deaths-injuries/
https://childrenshealthdefense.org/defender/clover-carroll-do-no-harm-covid-protocol-victims/
https://childrenshealthdefense.org/defender/nicole-charlotte-hardison-baby-remdesivir-covid/
https://childrenshealthdefense.org/defender/charlene-delfico-covid-protocol-death-otto-moring/
https://childrenshealthdefense.org/defender/constantine-gus-kotsanis-texas-covid-unvaccinated-died/
https://childrenshealthdefense.org/defender/pierre-kory-mary-beth-pfeiffer-usa-today-excess-deaths/
https://childrenshealthdefense.org/defender/grace-schara-covid-protocol-death/
https://childrenshealthdefense.org/defender/danielle-baker-pfizer-covid-vaccine-lawsuit/
https://childrenshealthdefense.org/defender/gail-seiler-cdc-covid-protocol/
https://childrenshealthdefense.org/defender/moderna-clinical-trial-documents-injuries/
https://childrenshealthdefense.org/defender/pfizer-biontech-covid-vaccine-placebo/
https://childrenshealthdefense.org/defender/confidential-eu-documents-deaths-pfizer-biontech-shots/
https://childrenshealthdefense.org/defender/jamie-kay-wylie-covid-protocol-death/
https://childrenshealthdefense.org/defender/steve-wenger-janssen-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/fda-cdc-covid-vaccine-myocarditis-safety-signal/
https://childrenshealthdefense.org/defender/ralph-marxen-jr-covid-hospital-death/
https://childrenshealthdefense.org/defender/united-kingdom-data-sharp-increase-excess-deaths/
https://childrenshealthdefense.org/defender/josephine-fillier-pfizer-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/terry-donohue-jenkins-pfizer-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/bbc-astrazeneca-vaccine-injuries-lawsuit/
https://childrenshealthdefense.org/defender/andre-cherry-moderna-vaccine-injury/
https://childrenshealthdefense.org/defender/covid-vaccine-injury-deaths-economic-damage/
https://childrenshealthdefense.org/defender/myocarditis-pfizer-covid-vaccine/
https://childrenshealthdefense.org/defender/karl-lauterbach-germany-covid-vaccine-injuries/
https://childrenshealthdefense.org/defender/iris-bryson-pfizer-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/covid-mask-vaccine-mandates-cities/
https://childrenshealthdefense.org/defender/moderna-spikevax-covid-vaccine-singapore-death/
https://childrenshealthdefense.org/defender/dedra-long-pfizer-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/adam-rowland-astrazeneca-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/infant-mortality-vaccine-doses/
https://childrenshealthdefense.org/defender/merck-covid-pill-virus-mutations/
https://childrenshealthdefense.org/defender/danielle-baker-pfizer-shots-transverse-myelitis/
https://childrenshealthdefense.org/defender/julie-gamble-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/aseem-malhotra-bbc-suspension-mrna-vaccines/
https://childrenshealthdefense.org/defender/robyn-forbes-pfizer-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/jake-holliday-pfizer-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/sandra-ortiz-pfizer-covid-vaccine-injury/
https://childrenshealthdefense.org/defender/family-vaccine-injuries-gardasil-covid/
https://childrenshealthdefense.org/defender/steven-ordonia-pfizer-covid-booster-vaccine-injuries/
https://childrenshealthdefense.org/defender/women-covid-vaccine-injuries-v-safe-data/
https://childrenshealthdefense.org/defender/cdc-data-covid-vaccine-injuries-vsafe-app/
https://childrenshealthdefense.org/defender/people-injured-covid-vaccines-stories/
https://childrenshealthdefense.org/defender/catherine-parker-covid-vaccine-injuries/
https://childrenshealthdefense.org/defender/covid-vaccine-injuries-global-campaign-canwetalkaboutit/
https://childrenshealthdefense.org/defender/deaths-injuries-pfizer-vaccine-trial-document-dump/
https://childrenshealthdefense.org/defender/pilots-injured-covid-vaccines-airlines-mandates/
https://childrenshealthdefense.org/defender/fda-novavax-covid-vaccine-heart-inflammation/
https://childrenshealthdefense.org/defender/fda-pfizer-documents-vaccine-adverse-events/
https://childrenshealthdefense.org/defender/pilots-injured-covid-vaccines-speak/
https://childrenshealthdefense.org/defender/pfizer-hired-600-people-vaccine-injury-reports/
https://childrenshealthdefense.org/defender/injury-covid-countermeasure-backlog-grows/



James Roguski
310-619-3055
JamesRoguski.substack.com/archive
ControlBloodSugarNaturally.com
I claim no copyright of any kind whatsoever, over any of my work, ever. Everyone is encouraged to copy any and all of it, in part, or in full, and use it for whatever purposes they wish. In fact, I would be delighted if someone were to copy this entire body of work. I encourage everyone to duplicate and mirror it in its entirety. I also encourage everyone to adapt and utilize the information in whatever manner they deem appropriate. No citation or other reference is requested or required. It would actually bring me great joy to see this information multiply exponentially and “go viral”.
All content is free to all readers.
All support is deeply appreciated.
-
AuthorPosts
- You must be logged in to reply to this topic.